Article contents
Improvement of luminescence properties of Ca0.8Zn0.2TiO3:Pr3+ prepared by hydrothermal method
Published online by Cambridge University Press: 28 August 2013
Abstract
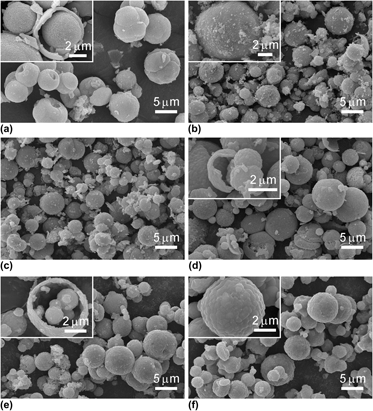
Red-emitting phosphor of Ca0.8Zn0.2TiO3:0.2 mol% Pr3+ was synthesized by the hydrothermal method with urea as a mineralizer. The crystalline structure, micromorphology, and luminescent properties of the resultant phosphor were investigated. Results show that elevated calcination temperature does not change the shape of particles that are hollow spheres with a shell thickness of 210–480 nm, and smaller particles are in the middle of the larger ones. The emission intensity at 612 nm originated from 1D2 → 3H4 transition of Pr3+ ions increases with the elevated calcination temperature due to a higher crystallinity. Excitation curves consist of two strong broad bands centered at about 330 and 380 nm and a weaker broad band range from 450 to 500 nm. The sample prepared by the hydrothermal method has better luminescent properties than that of its counterpart prepared by the solid-state method, especially the improvement of near-UV region (380 nm) excitation intensity.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 5
- Cited by


