People with severe mental illness (SMI) such as schizophrenia, schizoaffective disorder, bipolar affective disorder and depression with psychotic features have a reduction in life expectancy of up to 20 years, which has been labelled a ‘scandal of premature mortality’( Reference Thornicroft 1 ). Further, recent evidence shows that the mortality gap is widening( Reference Lawrence, Hancock and Kisely 2 ). Standard treatment for psychotic illness includes long-term antipsychotic medications (APM) combined with psychosocial interventions delivered by a multidisciplinary team( Reference McGorry, Killackey and Lambert 3 , Reference McGorry, Killackey and Yung 4 ).
APM can induce rapid weight gain with associated metabolic abnormalities( Reference Álvarez-Jiménez, González-Blanch and Crespo-Facorro 5 , Reference Correll, Manu and Olshanskiy 6 ). This rapid weight gain is most significant during the first 12 weeks after APM initiation, with a mean increase of 8 kg during this period, progressing to 12 kg over the first 2 years and 18 kg over the first 4 years of treatment( Reference Álvarez-Jiménez, González-Blanch and Crespo-Facorro 5 ). This weight gain contributes to the high rates of overweight and obesity in people with established SMI, with twice the rate of diabetes and rates of hypercholesterolaemia up to five times that of the general population( Reference Miller, Paschall and Svendsen 7 – Reference Hennekens, Hennekens and Hollar 9 ). Weight gain and cardiometabolic risk evolution associated with APM has been reviewed elsewhere( Reference Blanchard and Samaras 10 ). In addition to APM, factors contributing to weight gain include energy-dense, highly processed, high-salt diets( Reference Dipasquale, Pariante and Dazzan 11 ) and lower levels of physical activity( Reference Jerome, Rohm Young and Dalcin 12 ) compared with the general population, as well as higher rates of smoking and substance use( Reference Cooper, Mancuso and Borland 13 , Reference Bahorik, Newhill and Queen 14 ).
A high proportion of people receiving APM report considerably increased hunger and excessive night hunger, a decreased feeling of satiety and increased cravings for sweet foods and drinks( Reference Treuer, Hoffmann and Chen 15 , Reference Blouin, Tremblay and Jalbert 16 ). Additional eating behaviours in this population include high levels of fast food consumption, low food literacy, fast-eating syndrome and habits such as only eating one main meal daily( Reference Blouin, Tremblay and Jalbert 16 ).
Studies that have examined dietary intake of people with SMI have focused on people with chronic SMI, and are summarised in a systematic review that reported higher intakes of SFA and Na and lower intakes of fruit and fibre( Reference Dipasquale, Pariante and Dazzan 11 ). Studies of energy intake found higher intakes in those with SMI compared with the general population( Reference DeMyer, Ward and Lintzenich 17 – Reference Strassnig, Brar and Ganguli 19 ) and lower consumption of nutrient-dense foods such as vegetables, dairy products and legumes( Reference McCreadie 20 ).
Higher energy intake contributes to weight gain and diabetes and CVD risk( Reference Swinburn, Caterson and Seidell 21 ); this is compounded by high levels of sedentariness and lower BMR in people with SMI( Reference Cuerda, Velasco and Merchán-Naranjo 22 , Reference Sharpe, Byrne and Stedman 23 ). Given the dietary behaviours described above, interventions to reduce energy intake and improve diet quality, by increasing core foods such as fruits, vegetables, whole-grains, lean meats and dairy foods and by reducing discretionary foods such as processed foods high in kilojoules, SFA, added sugars, added salts and/or alcohol, could be key interventions to improve the physical health of people with SMI. Further, reducing Na intake is a valid method of reducing the risk of CVD( 24 ), and lowering the glycaemic load may reduce the risk of type 2 diabetes and CVD( Reference Barclay, Petocz and McMillan-Price 25 , Reference Mirrahimi, de Souza and Chiavaroli 26 ).
Despite evidence that lifestyle intervention improves the physical and biochemical measures in both early psychosis( Reference Alvarez-Jimenez, Gonzalez-Blanch and Vazquez-Barquero 27 , Reference Curtis, Watkins and Rosenbaum 28 ) and enduring SMI( Reference Skouroliakou, Giannopoulou and Kostara 29 – Reference Bruins, Jörg and Bruggeman 31 ), few studies have evaluated the impact of lifestyle interventions on nutritional intake. To date, no studies have assessed the impact of preventative nutrition interventions delivered to youth with first-episode psychosis (FEP) and the feasibility and acceptability of such interventions in this vulnerable population.
The aims of this study were as follows: (i) to report the dietary intake of youth with a FEP, (ii) to evaluate the feasibility and acceptability of a 12-week intensive dietitian-delivered nutrition intervention soon after initiation of APM and (iii) to evaluate its effectiveness in reducing energy intake and improving diet quality.
Methods
Design
Dietetic consultations were one component of the multidisciplinary Keeping the Body in Mind (KBIM) lifestyle and life skills intervention that was evaluated in a cluster-controlled study described elsewhere( Reference Curtis, Watkins and Rosenbaum 28 ). The intervention included an exercise physiologist-led physical activity programme and behavioural support, along with the dietetic intervention described below. All clients from one clinical service (Bondi Early Psychosis Program (EPP)) were offered the KBIM package of care. The comparison site (Liverpool Early Psychosis Intervention Program (EPIP)) received treatment as usual involving best-practice early intervention for FEP, without the KBIM lifestyle and life skills intervention. The study received ethics approval from the South Eastern Sydney Local Health District Human Research Ethics Committee (ref no: 13/040; LNR/13/POWH/85).
Participants/setting
All clients referred to the Bondi EPP, an early intervention service within a community healthcare centre, over a 12-month period, between February 2013 and February 2014, were invited to participate in the on-site nutrition intervention as part of the KBIM programme. The EPP clinical programme includes a multidisciplinary team focused on the recovery of normal life trajectory, using second-generation APM, other psychotropic medications as indicated (e.g. mood stabilisers and antidepressants), combined with community-based case-management and targeted behaviour and group programmes. Clients referred to the Liverpool EPIP served as a control group for the broader lifestyle intervention study; however, baseline and post-intervention nutritional intakes were not assessed at this site. Referral pathways for both EPP and EPIP included (i) acute inpatient units, (ii) general practitioner referral or (iii) transfer from another health service. Inclusion criteria were as follows: (i) first-episode psychosis, (ii) aged 15–25 years old and (iii) less than 1 month since commencement of APM. All participants met criteria for FEP before commencing APM. Those with more than 1 month of treatment with APM were excluded, as such treatment may already have resulted in significant weight gain and/or cardiometabolic disturbances. No eligible participants were prescribed medications to treat cardiometabolic complications including hypercholesterolaemia, type 2 diabetes and hypertension at commencement of the study. Psychiatric diagnoses were confirmed by treating psychiatrists during the course of treatment according to Diagnostic and Statistical Manual of Mental Disorder (DSM-5) criteria( 32 ).
Intervention
The dietitian offered weekly structured individual dietetic consultations for 12 weeks and focused on specific educational modules, utilising motivational interviewing and SMART (Specific, Measurable, Achievable, Relevant, Time Specific) goals( Reference Doran 33 ). A full assessment including medical and physical health history, together with a comprehensive diet history, was completed at the initial appointment. Subsequent consultations ranged from 30 to 60 min in duration and included a 24-h recall, diet history or food diary review plus education module(s) and goal setting. Educational modules delivered to all participants within the individual dietetic consultations targeted weight management, core foods and nutritional adequacy, and recommended serves as per the Australian Dietary Guidelines ( 34 ). Additional topics included meal structure, portion sizes, mindful eating, healthier eating and takeaway choices, label reading and budgeting. These subsequent topics were prioritised by the dietitian based on individual needs.
The dietitian facilitated a weekly group shopping tour and cooking session with assistance from a mental health nurse specialist. The dietitian and group members walked to the local supermarket to purchase ingredients, and then returned to the community centre to prepare lunch. During the weekly shopping tour and cooking session, education was provided and reinforced regarding nutritional requirements, healthy food choices, fresh food selection, food energy density, salt content, food safety as well as a variety of cooking skills. Both components of the nutrition intervention were offered weekly as part of standard care for people attending the Bondi EPP.
Participants also had the opportunity to take part in a weekly sports group and had access to an on-site gym supervised by an exercise physiologist.
Outcome measures
Energy intake, macronutrient and micronutrient intakes and glycaemic load were derived from a validated semi-quantitative/FFQ, the Dietary Questionnaire for Epidemiological Studies (DQES), developed by the Cancer Council of Victoria (Australia)( Reference Giles and Ireland 35 ). Following an explanation from the dietitian, participants self-completed the DQES based on intake over the last 3 months. Further assistance from the dietitian was provided if requested. Completed DQES forms were returned to the Cancer Council of Victoria for analysis, providing data on outcomes listed above. Feasibility and acceptability of the dietary consultations and group sessions were evaluated by a consumer survey (described elsewhere( Reference Teasdale, Rosenbaum and Watkins 36 )), through examination of attrition during the course of the intervention and through the number of consultations and weekly group sessions attended. The Australian Recommended Food Score (ARFS) was used to determine the proximity of core foods intake compared with the Australian Dietary Guidelines ( 34 ). The ARFS is a validated tool for use in the Australian population, producing an overall score, out of a possible 74 points, with reference to core food groups( Reference Collins, Watson and Burrows 37 , Reference Wirt and Collins 38 ). Discretionary food intake was measured separately by daily intake (g).
Statistical analysis methods
Baseline evaluation compared general dietary patterns including energy, fibre, Na, alcohol and discretionary food intakes with estimated energy requirements and national standards for recommended intakes( 39 ). BMR and estimated energy requirements were calculated using the Schofield equation( Reference Schofield 40 ). Energy under-reporting was classified as an energy intake (EI):BMR ratio<1·2. The pre-post evaluation assessed change in a range of nutritional factors that can impact on cardiometabolic health, including core food groups, discretionary foods, energy intake (kJ), Na intake (mg) and glycaemic load. Outcome measures were assessed using paired-sample t tests. Non-parametric χ 2 analysis was used for categorical variables. Partial η 2 (η p 2) effect sizes were calculated for mean change scores. Partial η 2 (η p 2) effect sizes were considered small at 0·10, medium at 0·25 and large at 0·40. Pearson’s correlations were calculated between mean change scores and mean number of contacts with clinicians. Repeated-measures ANOVA were calculated for mean change scores in anthropometric measures. Analyses were conducted using SPSS, version 22 package. A Bonferroni’s correction was applied to mean change scores assessed before and after intervention.
Results
During the recruitment period, twenty-seven participants completed baseline measures for the 12-week programme (twelve females and fifteen males, mean age=20·5 (sd 2·4) years). Clients were predominantly Caucasian (n 14, 52 %), and in decreasing frequency Asian (n 5, 19 %), Middle Eastern (n 3, 11 %), Indigenous Australians (n 2, 7 %), Maori/Pacific Islanders (n 2, 7 %) and African-Americans (n 1, 4 %). Diagnoses were as follows: schizophreniform disorder (n 12, 45 %), bipolar affective disorder (n 8, 30 %) schizoaffective disorder (n 2, 7 %), schizophrenia (n 2, 7 %), major depression with psychotic features (n 2, 7 %) and brief psychotic disorder (n 1, 4 %). A range of mood-stabiliser, antipsychotic and antidepressant medications was prescribed. In total, 50 % of the sample had unchanged medication for the duration of the intervention. Some changes in antipsychotic dosage occurred for the remaining participants (28 % decreased, 17 % increased). For one patient, APM was switched during the course of the intervention. Demographic, diagnostic and medication data are provided in Table 1.
Table 1 Characteristics of clients referred to the Bondi Early Psychosis Program and those who completed the intervention (Numbers and percentages; mean values and standard deviations)

At baseline, there was no difference between groups in mean weight (KBIM=67·6 (sd 13·2) kg, control=75·8 (sd 18·8) kg), BMI (KBIM=23·2 (sd 3·1) kg/m2, control=24·8 (sd 3·3) kg/m2) or waist circumference (KBIM=83·4 (sd 10·5) cm, control=83·0 (sd 9·1) cm) (all P-values>0·39). The multidisciplinary KBIM lifestyle and life skills intervention restricted antipsychotic-induced weight gain to a mean 1·1 kg (95 % CI −0·3, 2·5) during the 12-week intervention, whereas the control group had a mean weight gain of 7·8 kg (95 % CI 4·8, 10·7), F 1,28=24·4 (P<0·001). There was no change in waist circumference in the KBIM group, −0·3 (95 % CI −2·3, 1·6) cm, whereas the control group gained a mean 7·1 cm (95 % CI 4·8, 9·4), F 1,28=27·1 (P<0·001).
All participants completed the DQES at baseline. Baseline energy, discretionary food, Na, glycaemic load and diet quality intakes are described in Table 2. The mean reported energy intake was not significantly different from the estimated energy requirement (t(26)=−0·4, P=0·67). Energy was under-reported in 41 % (n 11, EI:BMR range 0·63–1·10). After accounting for under-reporting, reported energy intake, 11 385 (sd 3601) kJ, was significantly higher than the estimated energy requirement, 9550 (sd 1574) kJ, 1833 (sd 3067) kJ, t(15)=2·39, P=0·03.
Table 2 Baseline nutritional intake for twenty-seven participants (all participants), compared with sixteen participants (accounting for under-reporting), and recommended intakes

UL, Upper limit.
Two-thirds of the participants (nine females and nine males, mean age=20·0 (sd 2·1) years; range 17–24 years) remained engaged at the completion of the 12-week nutrition intervention. Reasons for disengagement included the following: five were unable to be contacted, three discontinued treatment with the mental healthcare service, one was re-admitted to inpatient care during intervention and one was incarcerated during the intervention period. There were no significant differences in age (P=0·5), sex (P=0·7), ethnicity (P=0·5) or diagnoses (P=0·2) between those who remained engaged in the programme and those who disengaged. There was no difference in rates of mood-stabiliser, antidepressant, antipsychotic prescription or antipsychotic polypharmacy between those who remained engaged in the programme and those who disengaged (P≥0·09).
The mean number of contacts with the dietitian was 8·2 (median=7·5, range 5–12) (individual consultations=6·1 (median=6·5, range 2–12) and shopping/cooking group=2·1 (median=2, range 0–5)). The mean number of contacts with the exercise physiologist was 10·9 (median=11, range 0–25). There was a trend to significance between the number of contacts with the dietitian and the number of contacts with the exercise physiologist (r 0·46, P=0·05).
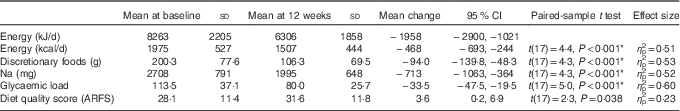
At completion of the 12-week programme, significant quantitative and qualitative changes were evident. Daily energy intake was reduced by 1956 kJ (95 % CI −2897, −1020, η p 2=0·51, P<0·001). This was reflected in reductions in carbohydrate (−54 g/d, P<0·001), sugar (−15 g/d, P=0·08), protein (−13 g/d, P=0·04) and fat (−22 g/d, P=0·001) intakes. Fibre intake was not significantly different before and after evaluations. Energy was under-reported in 50 % of the participants who remained engaged at the 12-week point (n 9; EI:BMR range 0·82–1·10). There was no relationship between energy underestimation at baseline and change in weight over the 12-week intervention (R 0·23, P=0·37) (Table 3).
Table 3 Mean change of eighteen participants from baseline to after the 12-week intervention (Mean values, standard deviations and 95 % confidence intervals)
ARFS, Australian Recommended Food Score.
* Statistically significant after applying Bonferroni’s correction.
After the 12-week programme, discretionary food intake reduced by 47 % (−94·0 g, 95 % CI −138·9, −48·3 g, η p 2=0·53, P<0·001), with associated reductions in Na intake, −713 mg (95 % CI −1063, −364, η p 2=0·52, P<0·001), and glycaemic load, −33·5 (95 % CI −47·5, −19·5, η p 2=0·60, P<0·001). Reductions in discretionary food intake correlated with reductions in overall energy intake (r 0·57, P=0·01). There was an increase in overall diet quality score (3·6 (95 % CI 0·3, 6·8, η p 2=0·23, P=0·03)), not statistically significant after applying Bonferroni’s correction for multiple comparisons.
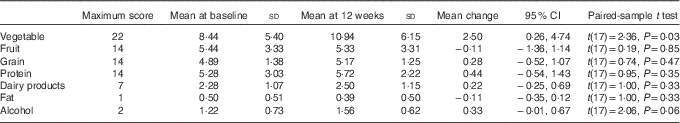
To further examine the components of diet quality, an analysis of diet quality subgroups was performed, with results shown in Table 4. The trend in improved diet quality score was driven by increased vegetable variety and frequency (2·4 (95 % CI 0·3, 1·0, P=0·031)).
Table 4 Mean change in diet quality score (Australian Recommended Food Score) by subgroup (Mean values, standard deviations and 95 % confidence intervals)
The correlation between greater contact with the dietitian and lower energy intake approached significance (r 0·45, P=0·06), and there was also a trend for the relationship between lower Na intake and number of contacts with the exercise physiologist (r 0·46, P=0·05). The magnitude of change in weight, waist circumference, discretionary food, glycaemic load and diet quality was not significantly correlated with contact with either the dietitian or the exercise physiologist (all r-values<0·45, all P-values>0·06).
Discussion
The present study demonstrated that (i) the dietary intake in youth early in their first episode of psychosis appears to be higher than required, driving the rapid weight gain seen with the commencement of APM, (ii) a 12-week dietary intervention was feasible and accepted by the majority of clients and (iii) the intervention was effective in improving dietary intake.
At baseline, reported energy intake did not differ significantly from estimated energy requirement. However, once accounted for under-reporting, reported energy intake was significantly higher than estimated energy, coinciding with frequent reporting of increased hunger in those commencing antipsychotic treatment, leading to rapid weight gain, similar to that seen in the control group. Future studies utilising weighed food records in the early stages of FEP treatment are required to confirm these findings.
A mean contact with the dietitian of 8·2, range 5–12, suggests that participants accepted the intervention. The individual consultations had a higher attendance rate compared with the cooking group. The low attendance rate in the cooking group may be explained in part by the session being offered at a set time each week, in contrast to the individual consultations, which were tailored to the participants’ availability.
The results of the intensive 12-week intervention suggest that overall intake of food in this target group can be improved, with poor-quality, energy-dense, discretionary foods replaced by core foods such as vegetables. The reduction in energy intake provides a means to attenuate antipsychotic-induced weight gain. Indeed, the multifaceted KBIM program demonstrated that weight gain could be prevented in the majority of youth commencing APM( Reference Curtis, Watkins and Rosenbaum 28 ).
The 12-week intervention also resulted in a reduction in Na intake, a proven risk factor for hypertension and heart disease. Na intake was reduced to within the Australian recommended upper limit of intake (<2300 mg/d)( 24 ).
Although intake of discretionary foods, which are characterised by high-sugar, high-fat, non-nutritious foods, decreased significantly, it was not possible to assess the change in added sugar consumption. Future studies should assess the impact of lifestyle intervention on added sugar with recent studies suggesting a link to CVD( Reference Yang, Zhang and Gregg 41 ) and recent changes in World Health Organization( 42 ) recommendations.
A recent study in enduring mental illness demonstrated that a telephone-delivered nutrition intervention reduced inactivity and improved fruit and vegetable intake, as well as overall diet quality in those with established illness( Reference Baker, Turner and Kelly 43 ). Importantly, the study also found significant increases in quality of life and global functioning. Although there were increases in participant vegetable intake in our study, fruit intake remained relatively unchanged. This may have been due to participants already meeting the recommendations of 2 servings of fruit/d at baseline assessment. In addition to monetary reimbursement and free fruit and vegetables boxes provided in Baker et al.’s( Reference Baker, Turner and Kelly 43 ) study, inclusion criteria specified only those consuming <7 fruit and vegetable serves/d. This may account, in part, for the large effect sizes seen in the study. Future studies assessing the cost-benefits of differing delivery methods would assist the design and delivery of nutrition interventions into standard practice.
At present, there are no recommended ranges for diet quality score when using the Australian Recommended Food Score. Mean diet quality scores in FEP clients were found to be lower than mean scores in established illness( Reference Baker, Turner and Kelly 43 ) and in Australian adult women of the general population( Reference Collins, Young and Hodge 44 ).
This study has a number of limitations, most notably the lack of age-, sex- and socio-demographic characteristic-matched controls. This was due to the pragmatic nature of this study, which aimed to evaluate the effectiveness of a real-world intervention offered to all clients of a service, such that randomisation to a comparison condition could not be undertaken. Although there is considerable evidence of decline in physical health during in the first 12 weeks of antipsychotic therapy( Reference Álvarez-Jiménez, González-Blanch and Crespo-Facorro 5 , Reference Correll, Robinson and Schooler 45 ), it is likely that nutritional status declines in parallel as weight gain and metabolic deterioration develop.
Although there was potential for a Hawthorne effect( Reference Roethlisberger, Dickson and Wright 46 ), there were no significant relationships between contact with the dietitian or exercise physiologist and dietary improvement. Similar to the majority of nutrition assessment tools, the responses to the dietary questionnaire were self-reported, potentially reducing accuracy and increasing risk of bias. Although the study had a relatively small sample size, the data showed clear improvements across a number of nutritional parameters.
In total, 67 % successfully completed 12 weeks of intensive intervention, demonstrating the feasibility and acceptability of the intervention. Youth with FEP can be difficult to engage, with 30 % attrition rates for a range of interventions offered in FEP services( Reference Doyle, Turner and Fanning 47 ), and these drop-out rates are similar to other lifestyle programmes conducted in people without SMI( Reference Evans, Newton and Higgins 48 , Reference Kwon, Choi and Bahk 49 ).
Clinicians delivering the intervention also noted a number of potential benefits as a result of the nutrition intervention that warrant further investigation. Having a nutrition programme embedded within the EPP was associated with increased attendance by clients at the community centre, allowing additional opportunities for client contact by mental health clinicians. Peer contact within the cooking group may have contributed to improved social skills and self esteem. Previous studies( Reference Jacka, Kremer and Berk 50 , Reference Kulkarni, Swinburn and Utter 51 ) have demonstrated that improved diet quality can increase psychosocial function in those with depression. Correlations between diet quality and psychosocial function scores in psychotic illness have not been evaluated, and future studies should assess the relationship between diet quality and symptomatology.
Conclusion
The integration of dietetic services into community early psychosis programmes is feasible and acceptable to youth with FEP with high attendance rates over a short-term period of 12 weeks. If dietary changes are sustained, intensive and early nutrition intervention may reduce future risk of cardiometabolic disease in SMI, helping meet the targets identified in the Healthy Active Lives international declaration for youth with psychosis (www.iphys.org.au). These targets aim to create a world where ‘young people experiencing psychosis have the same life expectancy and expectations of life as their peers who have not experienced psychosis’. Early intervention in the first episode of psychosis may have a greater long-term impact on the health of people with SMI and represents a greater return on clinician effort than our current practice of late addressing of poor metabolic health in established chronic mental illness.
Acknowledgements
The authors acknowledge staff and clients of the Bondi EPP for their assistance with this study.
The Mental Health and Drug & Alcohol Office (MHDAO), Ministry of Health, NSW, North Sydney, Australia provided funding. The MHDAO had no role in the design, analysis or writing of this article.
The KBIM programme was designed by J. C., P. B. W. and K. S., who also obtained funding. A. W., J. C. and K. S. provided clinical supervision. S. B. T. delivered the nutrition intervention and collected data. S. B. T. analysed the data with input from S. R., K. S. and P. B. W. S. B. T., S. R., A. W., K. S. and P. B. W. interpreted the data. S. B. T. led the manuscript preparation with input from corresponding authors. All authors contributed to the intellect of the manuscript.
The authors declare that there are no conflicts of interest.






