Cereal foods are an important contributor of carbohydrates and dietary fibre (DF) particularly in Northern Europe. In Denmark, bread accounts for approximately 17 % of dietary energy intake(Reference Fagt, Matthiessen and Biltoft-Jensen1) and, along with other cereal products, for approximately 60 % of the intake of DF(Reference Sørensen, Fagt, Møller, Cummings and Frølich2). The consumption pattern of bread in Denmark, however, has been changing during the last two decades, as the white type of bread based on refined wheat starch has replaced whole-grain cereal bread, particularly whole-grain rye bread(Reference Fagt, Matthiessen and Biltoft-Jensen1). This is a most regrettable development as whole-grain cereals are superior to refined starch in terms of DF, phytochemicals, vitamins and minerals(Reference Slavin3). Epidemiological studies have pointed to whole-grain cereal products as factors that may protect against CVD, some forms of cancer and type 2 diabetes(Reference Slavin3, Reference Jacobs, Andersen and Blomhoff4). Development of new ingredients high in DF and other nutrients is therefore most welcome.
An epidemiological study of Salmerón et al. (Reference Salmerón, Manson and Stampfer5) using prospective data from more than 65 000 women in the Nurses' Health Study pointed to the intake of cereal DF and the glycaemic load as factors that reduce the risk of type 2 diabetes. A meta-analysis based on data from forty-five relevant publications reached the same conclusion(Reference Livesey, Taylor and Hulshof6, Reference Livesey, Taylor and Hulshof7). Lower-glycaemic index (GI) diets reduced both fasting blood glucose and glycated protein independently of variance in available carbohydrates and DF intake(Reference Livesey, Taylor and Hulshof6). In addition, high DF intake further improved both blood glucose and glycated protein control. It was also found that insulin sensitivity was improved by lower GI, by higher DF intake in persons with type 2 diabetes, in overweight and obese persons, and in all studies combined(Reference Livesey, Taylor and Hulshof6). A lower GI with cereal-based foods is usually related to soluble DF, β-glucan and arabinoxylans (AX), as these polysaccharides may interfere with the digestion and absorption processes either by delaying gastric emptying or by slowing the uptake of nutrients from the small intestine(Reference Wood8). However, the efficiency of these polymers in the short-term regulation of GI varies depending on whether they are provided as isolates or naturally present in the food(Reference Lu, Walker and Muir9–Reference Juntunen, Laaksonen and Autio11). More recent studies have emphasised the importance of insoluble non-viscous DF for improving insulin sensitivity and glucose homoeostasis(Reference Robertson12, Reference Priebe, Wang and Weening13). Since insoluble DF has little or no influence on digestive events in the small intestine, the beneficial effects on insulin sensitivity and glucose homoeostasis must involve colonic metabolism(Reference Robertson12, Reference Priebe, Wang and Weening13). The metabolism of DF in the large intestine leads to the production of SCFA through colonic fermentation, and the main products are acetate, propionate and butyrate(Reference Bach Knudsen14). These SCFA are rapidly absorbed from the gut lumen, a fraction is metabolised by the colonic epithelium, but a significant part enters the portal and peripheral circulation(Reference Bach Knudsen14, Reference Bach Knudsen, Serena and Canibe15). Enhanced SCFA production, and butyrate in particular, has been linked to improved insulin sensitivity and glucose homoeostasis(Reference Robertson12, Reference Priebe, Wang and Weening13, Reference Robertson, Currie and Morgan16–Reference Gao, Yin and Zhang18). The mechanism(s) are not entirely clear, but appear to involve adipose tissue metabolism(Reference Robertson, Currie and Morgan16, Reference Robertson, Bickerton and Dennis17). So, the effects of whole-grain cereals on glucose homoeostasis are dual – soluble DF interferes with digestion and absorption events in the small intestine, while the insoluble DF executes its action through fermentation in the large intestine.
Low-GI foods have also been linked to improved postprandial glucose regulation even after the consumption of a consecutive ‘second meal’ that was standardised, i.e. without dietary manipulation(Reference Jenkins, Wolever and Taylor19, Reference Nilsson, Ostman and Preston20). Improved glucose homoeostasis was seen from breakfast to lunch or from a late evening meal to breakfast in human studies(Reference Nilsson, Ostman and Preston20). Inclusion of DF from barley, in particular, has shown promising effects(Reference Priebe, Wang and Weening13, Reference Nilsson, Ostman and Preston20). However, in spite of decreased postprandial plasma glucose, the secondary meal effect did not affect the plasma content of insulin, but was positively correlated with fasting levels of NEFA in plasma, suggesting that types and proportion of assimilated products (glucose, lactate and SCFA) deriving from carbohydrate interact with glucose homoeostasis(Reference Priebe, Wang and Weening13, Reference Nilsson, Ostman and Preston20).
A recent study with twenty ileal cannulated pigs fed the same breads as in the present study showed that the ileal starch digestibility was lower for the rye bread than the two wheat breads, and the variation in ileal viscosity across breads was related to the concentration and molecular weight of AX across diets(Reference LeGall, Serena and Jørgensen21, Reference LeGall, Eybye and Bach Knudsen22). It was also found that the flow rate of digesta through the small intestine was higher after the first daily meal than after the second daily meal, due to less gut fill following a long (14 h) fasting period overnight. The more rapid flow, however, had only a minor impact on the overall ileal digestibility of nutrients(Reference LeGall, Serena and Jørgensen21). We therefore hypothesised that dietary inclusion of DF from rye, which increases luminal viscosity and reduces starch digestibility, would decrease the glycaemic load and insulinaemic response. Furthermore, the experiment aimed at testing which role the fasting period before a meal plays on insulinaemic and glycaemic responses, because the fasting period before the first and second daily meals in human subjects differs greatly.
Materials and methods
Breads
The breads were made of standard white wheat flour (WFL), whole-wheat grain (WWG), wheat aleurone-rich flour (WAF) and rye aleurone-rich flour (RAF). The WFL and WWG breads were produced at Holstebro Technical College (Holstebro, Denmark), and the WAF and RAF breads were baked in a local bakery (Konditor-Bager Ørum, Denmark). The ingredients for the WFL bread were white wheat flour (Triticum aestivum L. cv. Tiger), purified wheat fibres (Vitacel R200; J. Rettenmaier and Söhne GmbH, Rosenberg, Germany), rapeseed oil, sugar, salt, wheat gluten (LCH A/S, Frederiksberg, Denmark) and yeast, while in WWG, WAF and RAF breads, whole-grain wheat (BFEL Karlsruhe, Germany), wheat aleurone-rich flour (Bühler AG, Uzwil, Switzerland) and rye aleurone-rich flour (Raisio plc, Raisio, Finland) replaced the white wheat flour and purified wheat fibre on an iso-DF basis. After production, the breads were cut into pieces and mixed. After weighing, meal portions were frozen at − 20°C and thawed immediately before consumption. The four breads were balanced with regard to starch, protein, fat, energy and DF, but they varied in DF characteristics (see Tables 1 and 2 for dietary ingredients and composition).
Table 1 Ingredients list of experimental diets
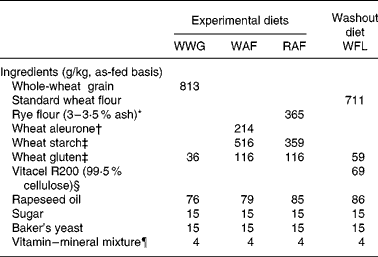
WWG, whole-wheat grain; WAF, wheat aleurone-rich flour; RAF, rye aleurone-rich flour; WFL, wheat flour.
* Raisio plc, Raisio, Finland.
† ASP02, Bühler AG, Uzwil, Switzerland.
‡ LCH A/S, Peter Bangs Vej 33, Frederiksberg, Denmark.
§ J. Rettenmaier and Söhne GmbH, Rosenberg, Germany.
¶ Supplying per kg diet: retinol, 660 μg; cholecalciferol, 12·5 μg; α-tocopherol, 30 mg; menadione, 11 mg; thiamin, 1 mg; riboflavin, 2 mg; d-pantothenic acid, 5·5 mg; niacin, 11 mg (available); biotin, 27·5 μg; cyanocobalamin, 11 μg; pyridoxine, 1·65 mg; Fe, 25 mg; Cu, 10 mg; Zn, 40 mg; Mn, 13·9 mg; Co, 0·15 mg; iodine, 0·01 mg; Se, 0·15 mg; and maize Ca2(PO4)3, K2PO3, NaCl and CaCO3 as a carrier (Solivit Mikro 106, Løvens Kemiske Fabrik, Vejen, Denmark).
Surgery, post-surgery care, housing and rearing were in compliance with Danish laws and regulations for the humane care and use of animals in research (The Danish Ministry of Justice, Animal Testing Act (Consolidation Act no. 726 of 9 September 1993 as amended by Act no. 1081 of 20 December 1995)). Furthermore, the Danish Animal Experimentation Inspectorate approved the study protocols and supervised the experiment. The health of the animals was monitored, and no serious illness was observed.
Daily feed allowance was 2·7 times of maintenance (2·7 × 460·2 kJ (110 kcal) of DE/kg0·75; NRC, 1998), which supplied 210 g DF/d. The daily ration was divided into three meals, and 40, 40 and 20 % of daily allowance was given at first (09.00 hours), second (14.00 hours) and third meals (19.00 hours) to mimic the diurnal variation in human intake of DF.
Animals
The pigs used in the study were from the swineherd at Aarhus University, Faculty of Agricultural Sciences, Foulum, Denmark. Six female LY pigs (56·5 (sem 1·8) kg) were included in the experiment, which was designed as a repeated 3 × 3 Latin square design. The pigs were adapted to the pen for 5 d, and then the animals were surgically fitted with a flow probe (Transonic, 20A probe, 20 mm; Transonic System, Inc., Ithaca, NY, USA) around the portal vein, a catheter in the portal vein and a catheter in the mesenteric artery. The surgical procedures have been described by Jørgensen et al. (Reference Jørgensen, Theil and Engberg23). After the surgery, the animals were allowed a 5–7 d recovery period before entering a 21 d experimental period (three consecutive experimental weeks). In each experimental week, the pigs were fed a washout diet (WFL) on days 1–3, and then the pigs were fed one of the three experimental breads on days 4–7. On day 7, fasting blood samples ( − 30 min before the first daily meal) were collected from the portal vein and the mesenteric artery, and then consecutive blood samples were drawn from the portal vein and the mesenteric artery at 0, 15, 30, 45, 60, 90, 120, 180, 240, 300 min (second daily meal), 315, 330, 345, 360, 390, 420, 480, 540 and 600 min relative to feeding the first daily meal. Blood samples were collected in Na-heparin vacutainers, and plasma was harvested; 9+4 ml of blood was collected at − 30 min (fasting), 9 ml of blood was collected at 15, 60, 120, 180, 240, 300, 315, 360, 420, 480, 540 and 600 min, whereas 2 ml of blood was collected at the remaining sampling times. Blood was replaced with saline. Four artery (Ia, IIa, IIIa and IVa) and four venous (Iv, IIv, IIIv and IVv) plasma samples were pooled from 9 ml blood samples to represent mean content of SCFA in blood in the periods 0–150, 150–300, 300–450 and 450–600 min after the morning feeding, respectively. Packed cell volume was monitored once per h and was always higher than 26 %. Blood flow in the portal vein was recorded immediately before blood collection as a mean of sixty recordings within a minute. Once a week, the pigs were weighed and received 400 mg of Fe supplement injected intramuscularly (Uniferon®; Pharmacosmos A/S, Holbæk, Denmark).
The pigs were kept individually in pens (2 × 2 m), and an elevated grid made of plastic covering half of the pen allowed the pigs to rest and stay dry. The pigs had access to water ad libitum, whereas no straw was supplied. The pigs were allowed 45 min to complete ingestion of a meal, whereupon the feed was removed forcing the pigs to eat up as fast as possible. Feed residues were collected and weighed, and DM content was analysed to allow correction of feed intake. Feed intake at a given meal was recorded to evaluate intake of starch, whereas intake during the last three meals was summed to evaluate the intake of DF.
Analytical methods
All chemical analyses of breads were performed in duplicate on freeze-dried materials. The DM was measured by drying to constant weight (mostly 20 h) at 103°C, and ash was analysed according to the AOAC method(24). Nitrogen was measured by DUMAS(Reference Hansen25), and protein was calculated as N × 6·25. Gross energy was determined with a LECO AC 300 automated calorimeter system 789-500 (LECO, St Joseph, MI, USA) (ISO 9831:1998). Fat was extracted with a monophasic mixture of chloroform, methanol and water as described by Bligh & Dyer(Reference Bligh and Dyer26) after hydrochloric acid hydrolysis.
Dietary contents of sugars (glucose, fructose and sucrose) and fructans were analysed as described by Larsson & Bengtsson(Reference Larsson and Bengtsson27), and starch and NSP were analysed as described by Bach Knudsen(Reference Bach Knudsen28). Klason lignin was measured as the sulphuric acid-insoluble residue as described by Theander & Åman(Reference Theander and Åman29). Pooled plasma samples were analysed for SCFA essentially as described by Brighenti(Reference Brighenti, Gullion, Amadò, Amaral-Collaco, Andersson, Asp, Bach Knudsen, Champ, Mathers, Robertson, Rowland and Van Loo30), using 2-ethyl butyrate (Fluka no. 03 190; Sigma Aldrich, St Louis, MO, USA) as an internal standard, rather than iso-valeric acid, because 2-ethyl butyrate is not produced by the microflora. All collected plasma samples were analysed for insulin by time-resolved fluoroimmunoassay as described by Løvendahl & Purup(Reference Løvendahl and Purup31) and for lactate and glucose according to standard procedures such as lactatoxidase, glucose hexokinase II and enzymatic colorimetric determination, respectively (Siemens Diagnostics® Clinical Methods for ADVIA 1650, Tarrytown, NY, USA). NEFA were determined using the Wako, NEFA C ACS-ACOD assay method (Wako Chemicals GmbH, Neuss, Germany). Lactate, glucose and NEFA analyses were performed on an ADVIA 1650 autoanalyzer (Siemens Healthcare Diagnostics).
Calculations
The net absorption of glucose, lactate and SCFA into the portal vein and apparent production of insulin were calculated from the porto-arterial differences and the portal flow measurements according to Rérat et al. (Reference Rérat, Vaissade and Vaugelade32) using the following equation:
where q is the amount of glucose, lactate, SCFA absorbed or insulin produced within the time period dt; C p is the concentration in the portal vein; C a is the concentration in the mesenteric artery; F is blood flow in the portal vein; and Q is the amount absorbed from t 0 to t n or insulin produced from t 0 to t n. The calculated insulin production can only be described in the present study as apparent because insulin has a pulsatile secretion, it is broken down by the liver and kidney and it has a variable half-life value (10–30 min).
The energy coefficients reported for glucose, lactate and specific SCFA(Reference Weast, Astle and Beyer33) were used when calculating portal absorption of energy derived from glucose, lactate and SCFA.
Statistics
Effects of breads, meal, time and their two-factor interactions were analysed as repeated measurements using the MIXED procedure of Statistical Analysis Software (SAS Institute Inc., Cary, NC, USA) as described by Littell et al. (Reference Littell, Milliken and Stroup34). Level of significance was detected at P ≤ 0·05, while a tendency was reported when P ≤ 0·10.
Plasma variables were analysed using the following normal mixed model:
where Y ijklmn is the dependent variable; αi is the bread (i = WWG, WAF or RAF); βj is the effect of meal (j = 1 or 2), which was also associated with length of time without feed before the meal (14 and 5 h, respectively); note that the postprandial phase following the third meal within a day was not studied; γk is the time after a meal (k = 15, 30, 45, 60, 90, 120, 180, 240 and 300 min); αβ ij, αγ ik and βγ jk are two-factor interaction terms; and δl is the week (l = 1, 2 or 3). The three terms ρm (m = pig 1,…,6), υilm and τilmn (n = meal 1 and 2) accounted for repeated measurements being performed on the same pig (ρm), on the same pig within period (υilm) and on the same pigs within period and meal (τilmn), respectively, whereas e ijklmn describes the random error. The covariance structure of τilmn was modelled using the spatial power option, which takes into account the different intervals between repeated measurements. Data on insulin concentrations in the mesenteric artery and the portal vein and apparent insulin production were transformed to ln(x) before performing the statistical analysis to obtain variance homogeneity. Plasma levels of SCFA (part of Table 4) and portal absorption of energy derived from glucose, lactate and SCFA (Table 5) were based on the pooled plasma samples. Thus, the above-mentioned model was applied, with the exception that γk describes the two absorptive phases after a meal (k = 1, 2 refer to 0–2·5 and 2·5–5 h post feeding, respectively), and a simple covariance structure of τilmn was assumed. If P>0·05 was found for bread × meal, meal × phase or bread × phase, the non-significant interaction terms were removed and the statistical model was reanalysed.
Results
Diets and dietary intake of nutrients
The diets were formulated to provide equal amounts of starch, DF, protein and energy, which was successfully accomplished (Table 2). The three diets used for intervention were characterised by a low content of cellulose (12–17 g/kg DM) and a high content of AX (50–62 g/kg DM). The content of AX was 22–24 % higher in WWG and WAF breads than in RAF bread, and the β-glucan content was double in WAF and RAF breads (8–9 g/kg DM) compared to WWG bread (4 g/kg DM). The dietary content of soluble DF was relatively high in RAF bread (29 g/kg DM), intermediate in WWG bread (23 g/kg DM) and low in WAF bread (13 g/kg DM). The WFL bread used as a washout diet had a high content of cellulose (66 g/kg DM) originating from the Vitacel fibre and low contents of soluble fibre (14 g/kg DM), AX (26 g/kg DM) and β-glucan (1 g/kg DM).
Table 2 Chemical composition of the experimental diets

WWG, whole-wheat grain; WAF, wheat aleurone-rich flour; RAF, rye aleurone-rich flour; WFL, wheat flour; AX, arabinoxylans.
The pigs had a lower intake of feed (P < 0·001) and DM (P = 0·02) when fed aleurone breads (WAF and RAF) as compared to WWG bread (Table 3). Consequently, the daily intake of starch was reduced by 14 and 11 % in pigs fed WAF and RAF breads, whereas the intake of digestible energy was reduced by 12 and 14 %, respectively. The daily intake of DF amounted to 196–211 g/d and was comparable among breads (P>0·10).
Table 3 Daily intake of feed, nutrients and energy on day 7 in the experimental week of pigs fed either whole-wheat grain (WWG), wheat aleurone-rich flour (WAF) or rye aleurone-rich flour (RAF) diets
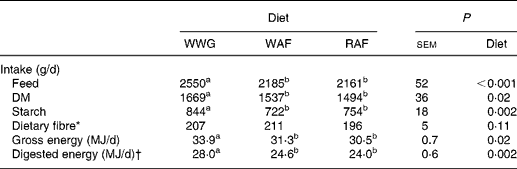
* Intake of fibre is determined based on the feed intake during the third meal on day 6 and the first two meals on day 7 (dietary fibre intake at these three meals acts as a substrate for fibre fermentation on day 7).
† Energy digestibility coefficients for the three experimental diets were determined by LeGall et al. (Reference LeGall, Serena and Jørgensen21) using twenty ileum-fistulated pigs.
Effects on metabolites in arterial and portal blood
In arterial blood, insulin was highest (P < 0·01) whereas propionate was lowest (P < 0·05) when the pigs were fed WWG breads compared to when the pigs were fed WAF and RAF breads (Table 4), whereas all other plasma metabolites were not affected by the dietary intervention in arterial blood. The dietary effects on plasma insulin and propionate found in arterial blood were consistent also in portal blood. Portal glucose concentration tended to be higher (P = 0·08) when the pigs were fed WWG breads, intermediate when fed RAF bread and lowest when fed WAF bread. In addition, portal butyrate was highest (P < 0·05) when the pigs were fed RAF bread, intermediate when fed WAF bread and lowest when fed WWG bread. Furthermore, portal concentrations of valerate (P < 0·001) and caproate (P < 0·05) were higher when the pigs were fed RAF bread compared to WAF and WWG breads. Concentrations of plasma insulin, glucose, lactate (P < 0·001), total SCFA (P < 0·10), acetate and iso-butyrate (P < 0·05) were affected by the time after feeding, both in arterial and in portal blood. In addition, the concentration of plasma valerate in arterial blood and concentrations of BCFA and iso-valerate in portal blood were affected by the time after feeding.
Table 4 Mean plasma concentrations of metabolites (0–10 h after the first daily meal) in the mesenteric artery and the portal vein in pigs fed either whole-wheat grain (WWG), wheat aleurone-rich flour (WAF) or rye aleurone-rich flour (RAF) breads

* sem is not an appropriate measure of variance because data were logarithmically transformed before data analysis. Instead, 95 % confidence limits are given in brackets.
Dietary effects on portal blood flow, insulin secretion, plasma NEFA and portal absorption of nutrients and energy
Portal blood flow was not affected by the three experimental diets (P>0·10; Table 5). The apparent insulin secretion was 51 % higher (P = 0·04) in pigs fed WWG bread as compared to RAF bread, whereas insulin secretion was intermediate in pigs fed WAF bread. No dietary effects were observed on portal NEFA concentration (P>0·10). Furthermore, the net absorption of glucose and lactate (P>0·10) was comparable among the three diets and amounted to 217–227 mmol/h of glucose and 24–27 mmol/h of lactate, respectively. Absorption of acetate was not affected by diet (P>0·10), whereas absorption of propionate was 46–58 % higher (P < 0·05) in pigs fed WAF and RAF breads compared to pigs fed WWG bread. Absorption of butyrate in pigs fed WAF and RAF breads increased by 34–41 % compared to pigs fed WWG bread. Absorption of valerate was 42 % higher (P < 0·01) and absorption of caproate was 244 % higher after RAF bread consumption (P < 0·01) as compared to consumption of WAF and WWG breads. Thus, in summary, the absorption of energy deriving from glucose and lactate was not influenced by the breads, whereas absorbed energy derived from SCFA (P < 0·05) was highest, intermediate and lowest in pigs fed RAF, WAF and WWG breads, respectively.
Table 5 Portal blood flow (l/min), apparent insulin secretion (nmol/h) and portal NEFA content (μeqv) and absorption of nutrients (mmol/h) and energy (kJ/h) derived from assimilated carbohydrates at fast and during two postprandial phases (I, 0–2·5 h; II, 2·5–5 h post feeding) in pigs fed either whole-wheat grain (WWG), wheat aleurone-rich flour (WAF) or rye aleurone-rich flour (RAF) breads at the first (09.00 hours) and second daily meals (14.00 hours) on day 7 of an experimental week

* Fasting levels were not included in the statistical meal × phase test. Significant differences between fasting and postprandial levels were obtained using Wald's test.
† sem is not an appropriate measure of variance because data were logarithmically transformed before data analysis. Instead, 95 % confidence limits are given in brackets.
Effects of meals and postprandial phases on portal blood flow, insulin secretion, plasma NEFA and portal absorption of nutrients and energy
Apparent insulin secretion after the first daily meal was twofold higher than after the second daily meal (8·8 v. 4·4 nmol/h, P < 0·001; Table 5), and in the first 2·5 h of the postprandial period, it was 1·5 times higher than in the following 2·5 h period (4·3 v. 2·8 nmol/h, P < 0·001). Plasma NEFA concentration was reduced postprandially compared to concentration at fasting, but the NEFA concentration was higher (P < 0·05) in the first 2·5 h period after the second daily meal than the first daily meal. Portal absorption of glucose and lactate was 21–28 % higher during the first 2·5 h of the postprandial period when compared to the consecutive 2·5 h period, whereas no meal effects were observed on absorption of glucose and lactate. In contrast, absorption of SCFA was comparable between the postprandial periods and differed between meals. Indeed, absorption of total and all individual SCFA was 35–53 % higher after consumption of the second daily meal than the first daily meal. Net portal absorption of energy changed with time after feeding and depended on the specific nutrient (Table 5). Glucose and lactate were predominantly absorbed during the first 2·5 h after feeding, and this pattern was consistent after both meals. In contrast, energy supplied to the portal vein as SCFA did not vary from the first 2·5 h to the next 2·5 h absorptive period within a meal, but the energy absorbed as SCFA was higher after the second daily meal than after the first daily meal. In total, the net portal absorption of energy originating from glucose, lactate and SCFA differed from the first to the second 2·5 h postprandial interval, whereas net portal absorption of energy in the 5 h following breakfast and lunch meals was comparable.
Postprandial dynamics of insulin secretion and absorption of glucose and lactate
Apparent insulin secretion (Fig. 1(a)) increased immediately after feeding, peaked 15–45 min after feeding and decreased thereafter. The apparent insulin secretion was inversely correlated to the net portal absorption of acetate (r − 0·24, P = 0·04), propionate (r − 0·39, P = 0·001) and butyrate (r − 0·35, P = 0·003), but was not correlated with net portal absorption of glucose (r 0·15, P>0·10). Like insulin secretion, the net portal absorption of glucose (Fig. 1(b)) and lactate (Fig. 1(c)) increased immediately after feeding. Glucose peaked after 45–60 min, whereas lactate peaked 60 min after feeding. The log of net portal glucose absorption plotted against time after feeding was linear in the interval 1–5 h as predicted by a first-order kinetic pool model, and slopes were − 4·5, − 4·8 and − 8·0 %/h for WWG, WAF and RAF breads, respectively (Fig. 2). The slope tended to be steeper (P = 0·07) for the RAF bread as compared to the two wheat breads. The log of net portal lactate absorption plotted against time after feeding was also linear in the interval 1–5 h after feeding with a slope of − 5·9 (sem 0·7) %/h and was not affected by diets (P>0·10).

Fig. 1 (a) Apparent insulin secretion (nmol/h): diet P = 0·04, time (T) P < 0·001, meal (M) P < 0·001, M × T P = 0·49, (b) glucose absorption (mmol/h): diet P = 0·84, time (T) P < 0·12, meal (M) P < 0·001, M × T P < 0·001 and (c) lactate absorption (mmol/h): diet P = 0·39, time (T) P < 0·001, meal (M) P = 0·09, M × T P < 0·001 in pigs fed whole-wheat grain (–△–), wheat aleurone-rich flour (–○–) or rye aleurone-rich flour (–●–) breads during the first 10 h after receiving the first daily meal (the second daily meal was given 5 h later, i.e. at time = 300 min).
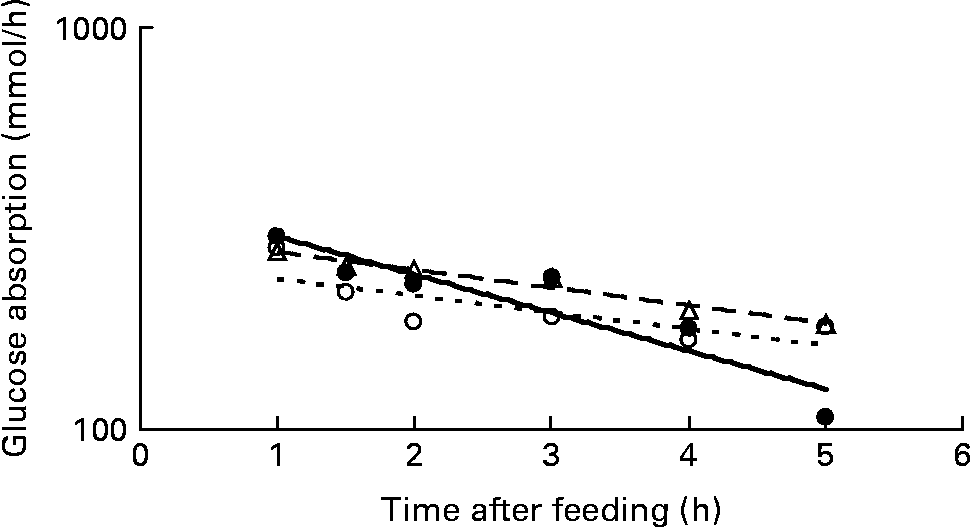
Fig. 2 Net portal absorption (log scale) of glucose in pigs fed whole-wheat grain (– –△– –), wheat aleurone-rich flour (- -○- -) or rye aleurone-rich flour breads (—●—).
Discussion
Dietary effects on glycaemic response
The net glucose absorption in the present study (217–227 mmol/h) was comparable during the postprandial period with that observed in other studies with growing pigs(Reference Bach Knudsen14, Reference Bach Knudsen, Jørgensen and Canibe35, Reference van der Meulen, Bakker and Smits36). However, the net glucose absorption in the present study was higher than that of observed when pigs were fed resistant starch(Reference van der Meulen, Bakker and Smits36, Reference van der Meulen, Bakker and Bakker37). The reason is most likely that cereal starch has an open structure which enables easy access for α-amylase to degrade starch(Reference Englyst, Kingman and Cummings38). In a parallel study with twenty ileum-fistulated pigs, we found an increased luminal viscosity and a lowered digestibility of starch in the small intestine when feeding RAF bread(Reference LeGall, Serena and Jørgensen21, Reference LeGall, Eybye and Bach Knudsen22), but the higher luminal viscosity and lower starch digestibility did not reduce glucose absorption in the present study. However, pigs consuming RAF bread in the present study seemed to respond differently compared to pigs fed WWG and WAF breads regarding the pattern of net portal glucose absorption. Indeed, the log of net portal flux of glucose indicated that starch was digested and glucose was absorbed faster when pigs were fed RAF breads. A likely explanation for this is that RAF bread contained no natural gluten(Reference Würsch and Bourne39).
Dietary effects on insulinaemic response
Consumption of RAF bread lowered the insulinaemic response compared to WWG bread, while WAF bread was intermediate. Several studies indicate that the presence of intact kernels is better than starch in attenuating the insulinaemic response(Reference Juntunen, Laaksonen and Autio11, Reference Juntunen, Niskanen and Liukkonen40), whereas the amount of DF is of less importance for the insulinaemic response. However, in the present study, the breads prepared from RAF and WAF did indeed attenuate the insulinaemic response to a greater extent than did WWG. It is logical to suggest that it was either the rye per se or bioactive components from the aleurone layer that were responsible for the attenuated insulinaemic response. In a recent study, rye breads based on endosperm or whole grain have been shown to lower insulinaemic responses and improve glycaemic profiles(Reference Rosen, Silva and Andersson41), which supports the notion that rye possesses health-promoting properties. Indeed, Rosen et al. (Reference Rosen, Silva and Andersson41) speculated that unknown bioactive components could be responsible for these beneficial features of rye. The other possible mechanism is that the aleurone fractions of both wheat and rye contain bioactive components that are able to improve insulin economy. No matter whether it is an unknown ‘rye factor’ or bioactive components in the aleurone fraction, the lowered insulinaemic response was not associated with an insufficient insulin signal. Indeed, numerically lower levels of plasma NEFA and higher glucose concentrations in the artery and the portal vein (P = 0·08) were observed along with the lower insulin secretion, and these findings suggest that the peripheral insulin sensitivity was improved in pigs fed RAF breads. A likely explanation for these results is that the affinity of the insulin receptors in muscle and fat tissues was improved by aleurone breads, which enhanced the overall insulin economy. In support of this, Fukagawa et al. (Reference Fukagawa, Anderson and Hageman42) demonstrated that DF originating from whole grains and vegetables was able to improve the peripheral sensitivity to insulin in young and elderly human subjects. It should be emphasised, however, that the lower feed intake observed when pigs were fed rye or wheat aleurone may have contributed to a minor extent in lowering the insulin response.
Butyrate – a possible candidate in regulating insulin secretion
Past studies have primarily focused on butyrate as an important nutrient for health and maintenance of the epithelium of the large intestine. More recently, studies have pointed to butyrate as an important nutrient in the release of hormones regulating energy homoeostasis(Reference Zhou, Hegsted and McCutcheon43), for improved insulin sensitivity(Reference Gao, Yin and Zhang18) and for increased energy expenditure in animals. Zhou et al. (Reference Zhou, Hegsted and McCutcheon43) reported that butyrate stimulated expression of peptide YY and proglucagon genes in caecum and colon cells in rats, thereby signalling satiety. Gao et al. (Reference Gao, Yin and Zhang18) found that butyrate increased the energy expenditure, increased the mitochondrial function in brown adipose tissue and improved the insulin sensitivity of obese rats. In the present study, the highest total absorption of SCFA and absorption of butyrate after consumption of RAF bread were associated with the lowest insulin secretion. Although the production of butyrate in cereal diets is linked to the digestion of AX in the gut(Reference Bach Knudsen and Lærke44), the higher production of butyrate with RAF diets can probably be associated with the high dietary contents of fructans, β-glucans and lower starch digestibility(Reference LeGall, Serena and Jørgensen21). A higher butyrate production may indeed be a central player for the insulinaemic response. In support of that, an increase in butyrate absorption at the second (compared to first) daily meal was also associated with a lower insulin secretion. In addition, animals whose colon has been removed surgically face problems in regulating the plasma level of glucose, as reviewed by Robertson(Reference Robertson12), which also suggests that butyrate may be an important metabolite for regulating insulin response. Indeed, an increasing body of evidence indicates that butyrate has multiple and adverse biological effects, and this aspect certainly deserves further attention.
First v. second daily meal
In the present study, a higher apparent insulin secretion was observed during the postprandial period after the first daily meal than after the second daily meal (P < 0·001). The attenuated insulin secretion following the second daily meal could not be attributed to an altered need to regulate blood glucose level because the meals supplied equal amounts of starch, and the total absorption of glucose was comparable in the postprandial periods following the first and second daily meals. Instead, the present results indicate that insulin secretion and thus insulin economy may vary considerably diurnally, and the applied feeding strategy may be a major contributor. The pigs in the present study were fed 40 % of the daily allowance at the first and second daily meals but 20 % at the third daily meal, and meals were provided with 5 h between servings. Thus, the pigs were in a catabolic state before the first daily meal, as indicated by a negative net portal flux of glucose (indicating mobilisation of glycogen or gluconeogenesis) and high NEFA levels (indicating mobilisation of fat). In contrast, the pigs were in an anabolic state when offered the second daily meal. In a previous study where pigs were fed three meals a day of equal size approximately every eighth hour, no profound insulin secretion was observed following consumption of the first daily meal(Reference Bach Knudsen, Serena and Kjaer45). But when pigs were fasted for 24 h and then fed a single meal, they responded by eliciting a profoundly higher insulin secretion, which was also associated with a low glucose clearance from the blood during the postprandial period(Reference Bach Knudsen, Serena and Kjaer45). Taken together, these results suggest that the insulin secretion, insulin sensitivity and thereby insulin economy are affected by the diurnal variation in energy supplied from the gastrointestinal tract. Hence, both a high-GI diet and large diurnal fluctuations in energy balance seem to be associated with higher insulin secretion. It should be underlined, though, that with the present pig model, it was not possible to investigate how the liver responded to altered portal energy supply following postprandial periods after breakfast and lunch meals.
In conclusion, insulin secretion was lowered in pigs fed RAF breads (compared to WWG bread) in spite of a similar intake of starch. Rye caused a higher luminal viscosity, which is expected to lower or slow the glucose absorption profile, which was not observed in the present study. The low insulin secretion following consumption of rye (and to a lesser extent following consumption of wheat aleurone) was associated with a high absorption of butyrate originating from bacterial fermentation in the caecum and colon, and an increasing body of evidence suggests a direct link between dietary stimulation of butyrate production and insulin economy.
Acknowledgements
The authors thank Helle Handll Christensen, Winnie Østergaard Thomsen, Kathrine Høirup Hansen and Lisbeth Märcher for technical assistance. The present research was financially supported by the European Commission in the Communities Sixth Framework Programme, Project HEALTHGRAIN (FOOD-CT-2005-514008). It reflects the authors' views, and the community is not liable for any use that may be made of the information contained in this publication. K. E. B. K. and A. S. were responsible for project development. P. K. T., J. H. and H. J. conducted the nutritional experiments in pigs. P. K. T. was responsible for the analyses and for generating statistical analyses. P. K. T. and K. E. B. K. were responsible for drafting the manuscript and P. K. T. is the corresponding author. There are no conflicts of interest.









