4 results
Molecular confirmation of pearl formation in arctic mussels (Mytilus edulis) caused by Gymnophallus bursicola (Odhner 1900) metacercariae
- Denis Benito, Urtzi Izagirre, Xabier Lekube, Beñat Zaldibar, Antonio Villalba, Xavier De Montaudouin, Guillemine Daffe, Manu Soto, Oihane Diaz de Cerio
-
- Journal:
- Parasitology / Volume 150 / Issue 11 / September 2023
- Published online by Cambridge University Press:
- 14 September 2023, pp. 1015-1021
-
- Article
-
- You have access Access
- Open access
- HTML
- Export citation
-
In recent field studies, suspected gymnophallid metacercariae were histologically located in the mantle of mussels from the Norwegian Sea. Mussels from the sites in which that infection was detected also presented abnormally high pearl numbers. It has been previously described that gymnophallid metacercariae could cause pearl formation processes in mussels, as a host reaction to encapsulate these metacercariae. Given the pathological host reaction these parasites elicit, a study was performed to identify gymnophallid metacercariae found in mussels collected from Tromsø at morphological and molecular level and to assess, by the use of molecular tools, the relationship between the parasite and the biological material inside the pearls. As a result, Gymnophallus bursicola metacercariae infecting Norwegian Mytilus edulis were identified according to morphological characters, along with the first 18S rDNA and COI sequences for this trematode species. In addition, parasite DNA from the core of the pearls was extracted and amplified for the first time, confirming the parasitological origin of these pearls. This procedure could allow identifying different parasitic organisms responsible for the generation of pearls in bivalves.
A numerical study of a variable-density low-speed turbulent mixing layer
- Antonio Almagro, Manuel García-Villalba, Oscar Flores
-
- Journal:
- Journal of Fluid Mechanics / Volume 830 / 10 November 2017
- Published online by Cambridge University Press:
- 02 October 2017, pp. 569-601
-
- Article
- Export citation
-
Direct numerical simulations of a temporally developing, low-speed, variable-density, turbulent, plane mixing layer are performed. The Navier–Stokes equations in the low-Mach-number approximation are solved using a novel algorithm based on an extended version of the velocity–vorticity formulation used by Kim et al. (J. Fluid Mech., vol 177, 1987, 133–166) for incompressible flows. Four cases with density ratios
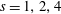 $s=1,2,4$ and 8 are considered. The simulations are run with a Prandtl number of 0.7, and achieve a
$s=1,2,4$ and 8 are considered. The simulations are run with a Prandtl number of 0.7, and achieve a 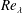 $Re_{\unicode[STIX]{x1D706}}$ up to 150 during the self-similar evolution of the mixing layer. It is found that the growth rate of the mixing layer decreases with increasing density ratio, in agreement with theoretical models of this phenomenon. Comparison with high-speed data shows that the reduction of the growth rates with increasing density ratio has a weak dependence with the Mach number. In addition, the shifting of the mixing layer to the low-density stream has been characterized by analysing one-point statistics within the self-similar interval. This shifting has been quantified, and related to the growth rate of the mixing layer under the assumption that the shape of the mean velocity and density profiles do not change with the density ratio. This leads to a predictive model for the reduction of the growth rate of the momentum thickness, which agrees reasonably well with the available data. Finally, the effect of the density ratio on the turbulent structure has been analysed using flow visualizations and spectra. It is found that with increasing density ratio the longest scales in the high-density side are gradually inhibited. A gradual reduction of the energy in small scales with increasing density ratio is also observed.
$Re_{\unicode[STIX]{x1D706}}$ up to 150 during the self-similar evolution of the mixing layer. It is found that the growth rate of the mixing layer decreases with increasing density ratio, in agreement with theoretical models of this phenomenon. Comparison with high-speed data shows that the reduction of the growth rates with increasing density ratio has a weak dependence with the Mach number. In addition, the shifting of the mixing layer to the low-density stream has been characterized by analysing one-point statistics within the self-similar interval. This shifting has been quantified, and related to the growth rate of the mixing layer under the assumption that the shape of the mean velocity and density profiles do not change with the density ratio. This leads to a predictive model for the reduction of the growth rate of the momentum thickness, which agrees reasonably well with the available data. Finally, the effect of the density ratio on the turbulent structure has been analysed using flow visualizations and spectra. It is found that with increasing density ratio the longest scales in the high-density side are gradually inhibited. A gradual reduction of the energy in small scales with increasing density ratio is also observed.
Contributors
-
- By Rose Teteki Abbey, K. C. Abraham, David Tuesday Adamo, LeRoy H. Aden, Efrain Agosto, Victor Aguilan, Gillian T. W. Ahlgren, Charanjit Kaur AjitSingh, Dorothy B E A Akoto, Giuseppe Alberigo, Daniel E. Albrecht, Ruth Albrecht, Daniel O. Aleshire, Urs Altermatt, Anand Amaladass, Michael Amaladoss, James N. Amanze, Lesley G. Anderson, Thomas C. Anderson, Victor Anderson, Hope S. Antone, María Pilar Aquino, Paula Arai, Victorio Araya Guillén, S. Wesley Ariarajah, Ellen T. Armour, Brett Gregory Armstrong, Atsuhiro Asano, Naim Stifan Ateek, Mahmoud Ayoub, John Alembillah Azumah, Mercedes L. García Bachmann, Irena Backus, J. Wayne Baker, Mieke Bal, Lewis V. Baldwin, William Barbieri, António Barbosa da Silva, David Basinger, Bolaji Olukemi Bateye, Oswald Bayer, Daniel H. Bays, Rosalie Beck, Nancy Elizabeth Bedford, Guy-Thomas Bedouelle, Chorbishop Seely Beggiani, Wolfgang Behringer, Christopher M. Bellitto, Byard Bennett, Harold V. Bennett, Teresa Berger, Miguel A. Bernad, Henley Bernard, Alan E. Bernstein, Jon L. Berquist, Johannes Beutler, Ana María Bidegain, Matthew P. Binkewicz, Jennifer Bird, Joseph Blenkinsopp, Dmytro Bondarenko, Paulo Bonfatti, Riet en Pim Bons-Storm, Jessica A. Boon, Marcus J. Borg, Mark Bosco, Peter C. Bouteneff, François Bovon, William D. Bowman, Paul S. Boyer, David Brakke, Richard E. Brantley, Marcus Braybrooke, Ian Breward, Ênio José da Costa Brito, Jewel Spears Brooker, Johannes Brosseder, Nicholas Canfield Read Brown, Robert F. Brown, Pamela K. Brubaker, Walter Brueggemann, Bishop Colin O. Buchanan, Stanley M. Burgess, Amy Nelson Burnett, J. Patout Burns, David B. Burrell, David Buttrick, James P. Byrd, Lavinia Byrne, Gerado Caetano, Marcos Caldas, Alkiviadis Calivas, William J. Callahan, Salvatore Calomino, Euan K. Cameron, William S. Campbell, Marcelo Ayres Camurça, Daniel F. Caner, Paul E. Capetz, Carlos F. Cardoza-Orlandi, Patrick W. Carey, Barbara Carvill, Hal Cauthron, Subhadra Mitra Channa, Mark D. Chapman, James H. Charlesworth, Kenneth R. Chase, Chen Zemin, Luciano Chianeque, Philip Chia Phin Yin, Francisca H. Chimhanda, Daniel Chiquete, John T. Chirban, Soobin Choi, Robert Choquette, Mita Choudhury, Gerald Christianson, John Chryssavgis, Sejong Chun, Esther Chung-Kim, Charles M. A. Clark, Elizabeth A. Clark, Sathianathan Clarke, Fred Cloud, John B. Cobb, W. Owen Cole, John A Coleman, John J. Collins, Sylvia Collins-Mayo, Paul K. Conkin, Beth A. Conklin, Sean Connolly, Demetrios J. Constantelos, Michael A. Conway, Paula M. Cooey, Austin Cooper, Michael L. Cooper-White, Pamela Cooper-White, L. William Countryman, Sérgio Coutinho, Pamela Couture, Shannon Craigo-Snell, James L. Crenshaw, David Crowner, Humberto Horacio Cucchetti, Lawrence S. Cunningham, Elizabeth Mason Currier, Emmanuel Cutrone, Mary L. Daniel, David D. Daniels, Robert Darden, Rolf Darge, Isaiah Dau, Jeffry C. Davis, Jane Dawson, Valentin Dedji, John W. de Gruchy, Paul DeHart, Wendy J. Deichmann Edwards, Miguel A. De La Torre, George E. Demacopoulos, Thomas de Mayo, Leah DeVun, Beatriz de Vasconcellos Dias, Dennis C. Dickerson, John M. Dillon, Luis Miguel Donatello, Igor Dorfmann-Lazarev, Susanna Drake, Jonathan A. Draper, N. Dreher Martin, Otto Dreydoppel, Angelyn Dries, A. J. Droge, Francis X. D'Sa, Marilyn Dunn, Nicole Wilkinson Duran, Rifaat Ebied, Mark J. Edwards, William H. Edwards, Leonard H. Ehrlich, Nancy L. Eiesland, Martin Elbel, J. Harold Ellens, Stephen Ellingson, Marvin M. Ellison, Robert Ellsberg, Jean Bethke Elshtain, Eldon Jay Epp, Peter C. Erb, Tassilo Erhardt, Maria Erling, Noel Leo Erskine, Gillian R. Evans, Virginia Fabella, Michael A. Fahey, Edward Farley, Margaret A. Farley, Wendy Farley, Robert Fastiggi, Seena Fazel, Duncan S. Ferguson, Helwar Figueroa, Paul Corby Finney, Kyriaki Karidoyanes FitzGerald, Thomas E. FitzGerald, John R. Fitzmier, Marie Therese Flanagan, Sabina Flanagan, Claude Flipo, Ronald B. Flowers, Carole Fontaine, David Ford, Mary Ford, Stephanie A. Ford, Jim Forest, William Franke, Robert M. Franklin, Ruth Franzén, Edward H. Friedman, Samuel Frouisou, Lorelei F. Fuchs, Jojo M. Fung, Inger Furseth, Richard R. Gaillardetz, Brandon Gallaher, China Galland, Mark Galli, Ismael García, Tharscisse Gatwa, Jean-Marie Gaudeul, Luis María Gavilanes del Castillo, Pavel L. Gavrilyuk, Volney P. Gay, Metropolitan Athanasios Geevargis, Kondothra M. George, Mary Gerhart, Simon Gikandi, Maurice Gilbert, Michael J. Gillgannon, Verónica Giménez Beliveau, Terryl Givens, Beth Glazier-McDonald, Philip Gleason, Menghun Goh, Brian Golding, Bishop Hilario M. Gomez, Michelle A. Gonzalez, Donald K. Gorrell, Roy Gottfried, Tamara Grdzelidze, Joel B. Green, Niels Henrik Gregersen, Cristina Grenholm, Herbert Griffiths, Eric W. Gritsch, Erich S. Gruen, Christoffer H. Grundmann, Paul H. Gundani, Jon P. Gunnemann, Petre Guran, Vidar L. Haanes, Jeremiah M. Hackett, Getatchew Haile, Douglas John Hall, Nicholas Hammond, Daphne Hampson, Jehu J. Hanciles, Barry Hankins, Jennifer Haraguchi, Stanley S. Harakas, Anthony John Harding, Conrad L. Harkins, J. William Harmless, Marjory Harper, Amir Harrak, Joel F. Harrington, Mark W. Harris, Susan Ashbrook Harvey, Van A. Harvey, R. Chris Hassel, Jione Havea, Daniel Hawk, Diana L. Hayes, Leslie Hayes, Priscilla Hayner, S. Mark Heim, Simo Heininen, Richard P. Heitzenrater, Eila Helander, David Hempton, Scott H. Hendrix, Jan-Olav Henriksen, Gina Hens-Piazza, Carter Heyward, Nicholas J. Higham, David Hilliard, Norman A. Hjelm, Peter C. Hodgson, Arthur Holder, M. Jan Holton, Dwight N. Hopkins, Ronnie Po-chia Hsia, Po-Ho Huang, James Hudnut-Beumler, Jennifer S. Hughes, Leonard M. Hummel, Mary E. Hunt, Laennec Hurbon, Mark Hutchinson, Susan E. Hylen, Mary Beth Ingham, H. Larry Ingle, Dale T. Irvin, Jon Isaak, Paul John Isaak, Ada María Isasi-Díaz, Hans Raun Iversen, Margaret C. Jacob, Arthur James, Maria Jansdotter-Samuelsson, David Jasper, Werner G. Jeanrond, Renée Jeffery, David Lyle Jeffrey, Theodore W. Jennings, David H. Jensen, Robin Margaret Jensen, David Jobling, Dale A. Johnson, Elizabeth A. Johnson, Maxwell E. Johnson, Sarah Johnson, Mark D. Johnston, F. Stanley Jones, James William Jones, John R. Jones, Alissa Jones Nelson, Inge Jonsson, Jan Joosten, Elizabeth Judd, Mulambya Peggy Kabonde, Robert Kaggwa, Sylvester Kahakwa, Isaac Kalimi, Ogbu U. Kalu, Eunice Kamaara, Wayne C. Kannaday, Musimbi Kanyoro, Veli-Matti Kärkkäinen, Frank Kaufmann, Léon Nguapitshi Kayongo, Richard Kearney, Alice A. Keefe, Ralph Keen, Catherine Keller, Anthony J. Kelly, Karen Kennelly, Kathi Lynn Kern, Fergus Kerr, Edward Kessler, George Kilcourse, Heup Young Kim, Kim Sung-Hae, Kim Yong-Bock, Kim Yung Suk, Richard King, Thomas M. King, Robert M. Kingdon, Ross Kinsler, Hans G. Kippenberg, Cheryl A. Kirk-Duggan, Clifton Kirkpatrick, Leonid Kishkovsky, Nadieszda Kizenko, Jeffrey Klaiber, Hans-Josef Klauck, Sidney Knight, Samuel Kobia, Robert Kolb, Karla Ann Koll, Heikki Kotila, Donald Kraybill, Philip D. W. Krey, Yves Krumenacker, Jeffrey Kah-Jin Kuan, Simanga R. Kumalo, Peter Kuzmic, Simon Shui-Man Kwan, Kwok Pui-lan, André LaCocque, Stephen E. Lahey, John Tsz Pang Lai, Emiel Lamberts, Armando Lampe, Craig Lampe, Beverly J. Lanzetta, Eve LaPlante, Lizette Larson-Miller, Ariel Bybee Laughton, Leonard Lawlor, Bentley Layton, Robin A. Leaver, Karen Lebacqz, Archie Chi Chung Lee, Marilyn J. Legge, Hervé LeGrand, D. L. LeMahieu, Raymond Lemieux, Bill J. Leonard, Ellen M. Leonard, Outi Leppä, Jean Lesaulnier, Nantawan Boonprasat Lewis, Henrietta Leyser, Alexei Lidov, Bernard Lightman, Paul Chang-Ha Lim, Carter Lindberg, Mark R. Lindsay, James R. Linville, James C. Livingston, Ann Loades, David Loades, Jean-Claude Loba-Mkole, Lo Lung Kwong, Wati Longchar, Eleazar López, David W. Lotz, Andrew Louth, Robin W. Lovin, William Luis, Frank D. Macchia, Diarmaid N. J. MacCulloch, Kirk R. MacGregor, Marjory A. MacLean, Donald MacLeod, Tomas S. Maddela, Inge Mager, Laurenti Magesa, David G. Maillu, Fortunato Mallimaci, Philip Mamalakis, Kä Mana, Ukachukwu Chris Manus, Herbert Robinson Marbury, Reuel Norman Marigza, Jacqueline Mariña, Antti Marjanen, Luiz C. L. Marques, Madipoane Masenya (ngwan'a Mphahlele), Caleb J. D. Maskell, Steve Mason, Thomas Massaro, Fernando Matamoros Ponce, András Máté-Tóth, Odair Pedroso Mateus, Dinis Matsolo, Fumitaka Matsuoka, John D'Arcy May, Yelena Mazour-Matusevich, Theodore Mbazumutima, John S. McClure, Christian McConnell, Lee Martin McDonald, Gary B. McGee, Thomas McGowan, Alister E. McGrath, Richard J. McGregor, John A. McGuckin, Maud Burnett McInerney, Elsie Anne McKee, Mary B. McKinley, James F. McMillan, Ernan McMullin, Kathleen E. McVey, M. Douglas Meeks, Monica Jyotsna Melanchthon, Ilie Melniciuc-Puica, Everett Mendoza, Raymond A. Mentzer, William W. Menzies, Ina Merdjanova, Franziska Metzger, Constant J. Mews, Marvin Meyer, Carol Meyers, Vasile Mihoc, Gunner Bjerg Mikkelsen, Maria Inêz de Castro Millen, Clyde Lee Miller, Bonnie J. Miller-McLemore, Alexander Mirkovic, Paul Misner, Nozomu Miyahira, R. W. L. Moberly, Gerald Moede, Aloo Osotsi Mojola, Sunanda Mongia, Rebeca Montemayor, James Moore, Roger E. Moore, Craig E. Morrison O.Carm, Jeffry H. Morrison, Keith Morrison, Wilson J. Moses, Tefetso Henry Mothibe, Mokgethi Motlhabi, Fulata Moyo, Henry Mugabe, Jesse Ndwiga Kanyua Mugambi, Peggy Mulambya-Kabonde, Robert Bruce Mullin, Pamela Mullins Reaves, Saskia Murk Jansen, Heleen L. Murre-Van den Berg, Augustine Musopole, Isaac M. T. Mwase, Philomena Mwaura, Cecilia Nahnfeldt, Anne Nasimiyu Wasike, Carmiña Navia Velasco, Thulani Ndlazi, Alexander Negrov, James B. Nelson, David G. Newcombe, Carol Newsom, Helen J. Nicholson, George W. E. Nickelsburg, Tatyana Nikolskaya, Damayanthi M. A. Niles, Bertil Nilsson, Nyambura Njoroge, Fidelis Nkomazana, Mary Beth Norton, Christian Nottmeier, Sonene Nyawo, Anthère Nzabatsinda, Edward T. Oakes, Gerald O'Collins, Daniel O'Connell, David W. Odell-Scott, Mercy Amba Oduyoye, Kathleen O'Grady, Oyeronke Olajubu, Thomas O'Loughlin, Dennis T. Olson, J. Steven O'Malley, Cephas N. Omenyo, Muriel Orevillo-Montenegro, César Augusto Ornellas Ramos, Agbonkhianmeghe E. Orobator, Kenan B. Osborne, Carolyn Osiek, Javier Otaola Montagne, Douglas F. Ottati, Anna May Say Pa, Irina Paert, Jerry G. Pankhurst, Aristotle Papanikolaou, Samuele F. Pardini, Stefano Parenti, Peter Paris, Sung Bae Park, Cristián G. Parker, Raquel Pastor, Joseph Pathrapankal, Daniel Patte, W. Brown Patterson, Clive Pearson, Keith F. Pecklers, Nancy Cardoso Pereira, David Horace Perkins, Pheme Perkins, Edward N. Peters, Rebecca Todd Peters, Bishop Yeznik Petrossian, Raymond Pfister, Peter C. Phan, Isabel Apawo Phiri, William S. F. Pickering, Derrick G. Pitard, William Elvis Plata, Zlatko Plese, John Plummer, James Newton Poling, Ronald Popivchak, Andrew Porter, Ute Possekel, James M. Powell, Enos Das Pradhan, Devadasan Premnath, Jaime Adrían Prieto Valladares, Anne Primavesi, Randall Prior, María Alicia Puente Lutteroth, Eduardo Guzmão Quadros, Albert Rabil, Laurent William Ramambason, Apolonio M. Ranche, Vololona Randriamanantena Andriamitandrina, Lawrence R. Rast, Paul L. Redditt, Adele Reinhartz, Rolf Rendtorff, Pål Repstad, James N. Rhodes, John K. Riches, Joerg Rieger, Sharon H. Ringe, Sandra Rios, Tyler Roberts, David M. Robinson, James M. Robinson, Joanne Maguire Robinson, Richard A. H. Robinson, Roy R. Robson, Jack B. Rogers, Maria Roginska, Sidney Rooy, Rev. Garnett Roper, Maria José Fontelas Rosado-Nunes, Andrew C. Ross, Stefan Rossbach, François Rossier, John D. Roth, John K. Roth, Phillip Rothwell, Richard E. Rubenstein, Rosemary Radford Ruether, Markku Ruotsila, John E. Rybolt, Risto Saarinen, John Saillant, Juan Sanchez, Wagner Lopes Sanchez, Hugo N. Santos, Gerhard Sauter, Gloria L. Schaab, Sandra M. Schneiders, Quentin J. Schultze, Fernando F. Segovia, Turid Karlsen Seim, Carsten Selch Jensen, Alan P. F. Sell, Frank C. Senn, Kent Davis Sensenig, Damían Setton, Bal Krishna Sharma, Carolyn J. Sharp, Thomas Sheehan, N. Gerald Shenk, Christian Sheppard, Charles Sherlock, Tabona Shoko, Walter B. Shurden, Marguerite Shuster, B. Mark Sietsema, Batara Sihombing, Neil Silberman, Clodomiro Siller, Samuel Silva-Gotay, Heikki Silvet, John K. Simmons, Hagith Sivan, James C. Skedros, Abraham Smith, Ashley A. Smith, Ted A. Smith, Daud Soesilo, Pia Søltoft, Choan-Seng (C. S.) Song, Kathryn Spink, Bryan Spinks, Eric O. Springsted, Nicolas Standaert, Brian Stanley, Glen H. Stassen, Karel Steenbrink, Stephen J. Stein, Andrea Sterk, Gregory E. Sterling, Columba Stewart, Jacques Stewart, Robert B. Stewart, Cynthia Stokes Brown, Ken Stone, Anne Stott, Elizabeth Stuart, Monya Stubbs, Marjorie Hewitt Suchocki, David Kwang-sun Suh, Scott W. Sunquist, Keith Suter, Douglas Sweeney, Charles H. Talbert, Shawqi N. Talia, Elsa Tamez, Joseph B. Tamney, Jonathan Y. Tan, Yak-Hwee Tan, Kathryn Tanner, Feiya Tao, Elizabeth S. Tapia, Aquiline Tarimo, Claire Taylor, Mark Lewis Taylor, Bishop Abba Samuel Wolde Tekestebirhan, Eugene TeSelle, M. Thomas Thangaraj, David R. Thomas, Andrew Thornley, Scott Thumma, Marcelo Timotheo da Costa, George E. “Tink” Tinker, Ola Tjørhom, Karen Jo Torjesen, Iain R. Torrance, Fernando Torres-Londoño, Archbishop Demetrios [Trakatellis], Marit Trelstad, Christine Trevett, Phyllis Trible, Johannes Tromp, Paul Turner, Robert G. Tuttle, Archbishop Desmond Tutu, Peter Tyler, Anders Tyrberg, Justin Ukpong, Javier Ulloa, Camillus Umoh, Kristi Upson-Saia, Martina Urban, Monica Uribe, Elochukwu Eugene Uzukwu, Richard Vaggione, Gabriel Vahanian, Paul Valliere, T. J. Van Bavel, Steven Vanderputten, Peter Van der Veer, Huub Van de Sandt, Louis Van Tongeren, Luke A. Veronis, Noel Villalba, Ramón Vinke, Tim Vivian, David Voas, Elena Volkova, Katharina von Kellenbach, Elina Vuola, Timothy Wadkins, Elaine M. Wainwright, Randi Jones Walker, Dewey D. Wallace, Jerry Walls, Michael J. Walsh, Philip Walters, Janet Walton, Jonathan L. Walton, Wang Xiaochao, Patricia A. Ward, David Harrington Watt, Herold D. Weiss, Laurence L. Welborn, Sharon D. Welch, Timothy Wengert, Traci C. West, Merold Westphal, David Wetherell, Barbara Wheeler, Carolinne White, Jean-Paul Wiest, Frans Wijsen, Terry L. Wilder, Felix Wilfred, Rebecca Wilkin, Daniel H. Williams, D. Newell Williams, Michael A. Williams, Vincent L. Wimbush, Gabriele Winkler, Anders Winroth, Lauri Emílio Wirth, James A. Wiseman, Ebba Witt-Brattström, Teofil Wojciechowski, John Wolffe, Kenman L. Wong, Wong Wai Ching, Linda Woodhead, Wendy M. Wright, Rose Wu, Keith E. Yandell, Gale A. Yee, Viktor Yelensky, Yeo Khiok-Khng, Gustav K. K. Yeung, Angela Yiu, Amos Yong, Yong Ting Jin, You Bin, Youhanna Nessim Youssef, Eliana Yunes, Robert Michael Zaller, Valarie H. Ziegler, Barbara Brown Zikmund, Joyce Ann Zimmerman, Aurora Zlotnik, Zhuo Xinping
- Edited by Daniel Patte, Vanderbilt University, Tennessee
-
- Book:
- The Cambridge Dictionary of Christianity
- Published online:
- 05 August 2012
- Print publication:
- 20 September 2010, pp xi-xliv
-
- Chapter
- Export citation
Perkinsosis in molluscs: A review
- Antonio Villalba, Kimberly S. Reece, M. Camino Ordás, Sandra M. Casas, Antonio Figueras
-
- Journal:
- Aquatic Living Resources / Volume 17 / Issue 4 / October 2004
- Published online by Cambridge University Press:
- 15 December 2004, pp. 411-432
- Print publication:
- October 2004
-
- Article
- Export citation
-
The genus Perkinsus includes protistan parasites infecting marine molluscs throughout the world, some of which are associated with mass mortalities. Life cycle involves vegetative proliferation within the host, by which a cell named trophozoite undergoes successive bipartitioning. Other stages have been observed in vitro or in vivo, depending on the species: hypnospore, zoosporangium and zoospore. Molecular taxonomy supports a close affinity between dinoflagellates and Perkinsus spp. Six species of Perkinsus are currently considered valid: P. marinus, P. olseni, P. qugwadi, P. chesapeaki, P. andrewsi and P. mediterraneus. Histology and, above all, incubation of host tissues in Ray's fluid thioglycollate medium (RFTM) are classic diagnostic methods. In addition, more sensitive and quicker molecular diagnostic techniques based on either immunoassays or PCR have been developed for Perkinsus spp. Epizootiological studies have shown a marked influence of water temperature and salinity on P. marinus infection in oysters Crassostrea virginica, thus determining parasite geographical range and temporal disease dynamics (seasonality). In vitro cultures have been established for four Perkinsus spp. Immune response to infection varies depending on host and involves phagocytosis or encapsulation of the parasite cells by host haemocytes. A polypeptide is secreted by clam Tapes philippinarum haemocytes that could kill the parasite. In vitro cultured P. marinus cells secrete proteases that are likely involved in degradation of host tissues. P. marinus can suppress the toxic oxygen radicals produced by host haemocytes. In addition to host death, sublethal effects caused by Perkinsus spp. (reduction of fecundity, growth, and condition) may have significant ecological and economic implications. Various strategies have been assayed to mitigate the consequences of P. marinus epizootics on the oyster industry: modifications of management/culture procedures, selective breeding to obtain resistant oyster strains, and the use of triploid oysters and allochthonous oyster species. Some chemotherapeutants have been proved to inhibit or kill parasite cells in vitro.