Mona Suleiman Awarded Inaugural Irish Society for Parasitology William C. Campbell Award 2022
The Irish Society for Parasitology organises a vibrant annual meeting for parasitologists to come together and share their research and build collaborations. The 2022 Annual Meeting of the Irish Society for Parasitology was hosted at the National University of Ireland Galway, as well as online for virtual attendees. Each year Cambridge University Press (the publishers of Parasitology) sponsor The Prof. William C. Campbell Postgraduate Award, so named after the Nobel Laureate scientist who was involved in the discovery of the ivermectins, a class of anthelmintics. The award is given to the postgraduate student who delivers the best presentation at the annual meeting. This year, Mona Suleiman, who is doing her PhD at the University of Bath, was the winner of the award. The competition this year was high, but Mona’s presentation stood out for the fundamental impacts of her research that were explained in a very comprehensible way. Mona introduces herself and her research below.
Although it is very cliché to say this, the first time I fell in love with parasites was during my first parasitology lecture where we were identifying the life cycle of parasitic worms and studying their role in health and disease. That is when I decided to start my career in parasitology and focus on parasitic worms and how they interact with their host. I am currently in the fourth and final year of my PhD investigating the role of non-coding RNAs expressed and secreted by the gastrointestinal parasitic nematode Strongyloides,within the University of Bath in the Hunt Lab. Strongyloides species are parasitic nematodes (worms) which infect an estimated 600 million people globally causing chronic morbidity and, more rarely, fatal disseminated strongyloidiasis (invasion of numerous organs). They also infect animals causing substantial economic loss in livestock practices. Understanding how these parasites infect their host is an important step towards developing novel ways of controlling and treating nematode infections.
The fascinating aspect of Strongyloides is that the life cycle includes genetically identical parasitic and free-living female adult stages. Direct comparison between these two adult stages can therefore uncover genetic features associated with parasitism and the parasitic lifecycle, not found in other parasites. Over the past three years of my PhD, I have been working on three different projects, one of which I have recently just finished and submitted as a first-author paper. This project was also presented in the Irish Society of Parasitology. In this study, we especially focused on the regulation of transposable element (TE) by small RNAs (sRNAs) in Strongyloides. TEs are selfish mobile DNA sequences that move around the genome from one location to another, causing random mutations. They have played an important role in the evolution of organisms but have also caused detrimental defects to the genome and require tight regulation. To control and regulate TEs, many organisms have developed a specific pathway of sRNAs called PIWI interacting RNAs (piRNAs). What’s interesting is that most nematode species, including Strongyloides are missing the piRNA pathway that regulate TEs. The aim of my project was therefore to answer the question, “how do parasitic nematodes regulate TEs and stop them from taking over the genome and still survive as parasitic species that cause diseases in millions of humans and animals?”.
Our results have shown that both lifecycle stages (parasitic and free-living female) have a diverse set of sRNAs. Interestingly however, we also found a specific set of sRNAs named 21-22Us that are only found in the parasitic stage of Strongyloides. We predict that the 21-22Us target and regulate their own TEs in the genome. Although Strongyloides has lost the piRNA pathway, the 21-22Us show striking resemblance to the piRNAs of C. elegans or Drosophila, including the way they are arranged in the genome and composition of the RNA sequences. We predict that these 21-22U piRNA-like sRNAs may be directly related to parasitism or feature associated with the parasitic lifecycle. In the future, we hope to carry out further experiments to show the importance of these 21-22Us and their potential in aiding in diagnostics and treatment of parasitic nematode infections.
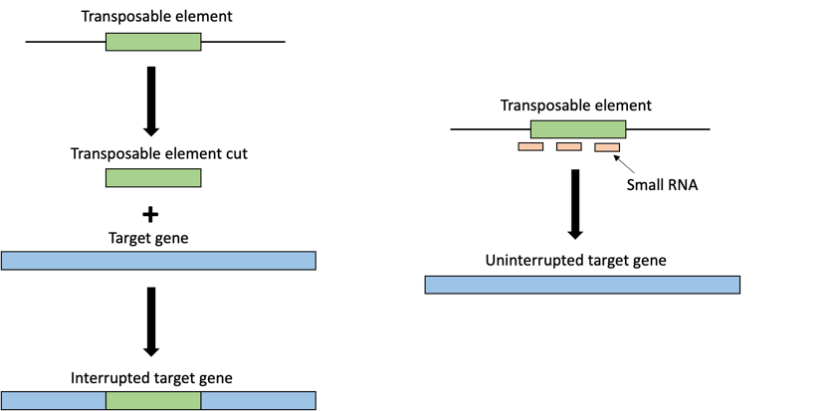
Schematic representation of a transposable element (TE) (green) moving from one part of the genome to another interrupting a target gene (blue) and therefore causing mutations or altering the target protein formed from the gene. In the presence of small RNAs (sRNAs) (orange), specifically piRNAs, TEs are targeted and regulated.
Currently and for the remainder of my PhD, I am writing my second and third manuscript regarding the role of sRNA secretion in exosomes by Strongyloides and how that affects the host gastrointestinal gene expression. We have found some intriguing results that show how exosome-like vesicles containing sRNAs play an important role in establishing parasitism by invading host cells and evading the immune system. We will also try to investigate how to use these as potential parasitology prognostic markers of disease progression and biomarkers for diagnostics. I am hugely thrilled to have presented my work to the Irish Society for Parasitology and thank them for giving me this award. I hope to continue attending the conference in the future and present the other projects I am currently working on.
Read the full open access article here: ‘Updates on Parasitology and adopting a Gold Open Access model of production‘