Better ways of measuring castrating parasites in the gonads of molluscs
The paper “An efficient photograph-based quantitative method for assessing castrating trematode parasites in bivalve molluscs” published in Parasitology, is available open access.
Trematodes (parasitic flatworms) frequently infect the gonad of molluscs such as mussels and snails, but no good methods exist for measuring the level of infection. There are important reasons why it may be useful to know how much of the mollusc gonad is filled with trematodes. For example, schistosomiasis is a disease caused by a trematode; humans get infected by trematode ‘cercariae’ (little swimming organisms) that are released from aquatic snails – the more trematodes a snail has living in its gonads, the more infective stages will be released. Schistosomiasis kills about 200,000 people per year, and 229 million people required preventative treatment in 2018: therefore, knowing the infection trends in snails could be useful in managing the disease. Another reason is that, at high levels of infection, the mollusc can be castrated by all the trematodes living in its gonad. This means that their populations could decrease, which could have wider effects on the environment. For example, many molluscs fulfil many important functions like water filtration – the entire volume of the River Cam in England is filtered through freshwater mussels every two weeks! These important ecosystem services could be lost if infection rates are not properly understood.
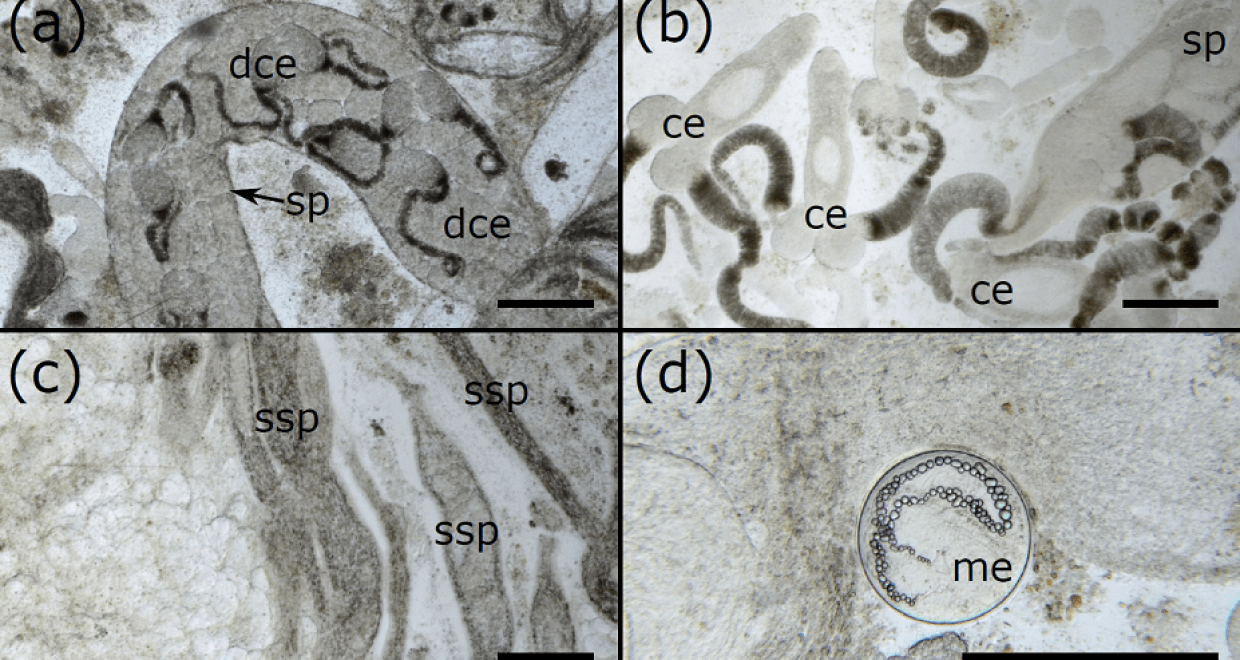
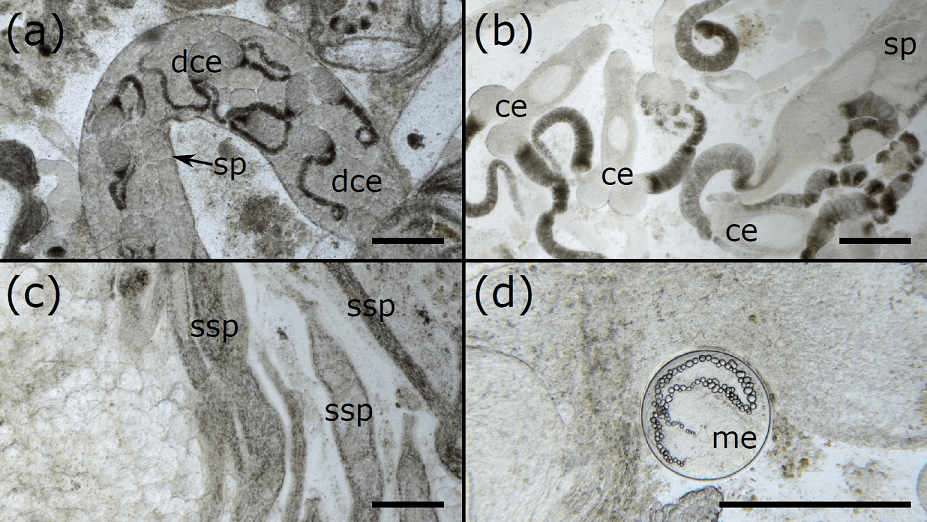
Currently, there is no clear method for measuring the level of infection in molluscs, with assessments usually being binary (e.g. ‘infected’ or ‘not infected’) or based on subjective judgements (e.g. ‘low infection’ or ‘high infection’). In our paper, we show that by taking small samples from the gonad, photographing them under a microscope, and then digitally processing the photographs, you can quickly and reliably estimate the percentage of the mollusc gonad filled with trematode tissue. Further, we showed that the number of photos required to estimate the level of infection varies depending on the size of the mollusc, but even the biggest mussels in our study only required 15 photographs, making this method very efficient. By providing a clear, simple, and repeatable method, we can easily compare the results of different studies, and can assess infection levels with confidence. We can also clearly see and quantify the development stages of the trematode – distinguishing between different types of sporocyst (asexually-produced tubes that wiggle through the gonad) and cercariae (the infective stages that will go on to infect the next host in the life cycle) that are produced inside the sporocyst tubes. We hope our method can facilitate high-quality studies on trematodes and their life cycles and help in a range of areas such as conservation and human health.
The paper “An efficient photograph-based quantitative method for assessing castrating trematode parasites in bivalve molluscs“, by Joshua I. Brian and David C. Aldridge, published in Parasitology, is available open access.
Joshua I. Brian, David C. Aldridge; Department of Zoology, University of Cambridge