Mania is a clearly defined condition that can present in different levels of severity, varying from the mild (hypomanic) to the florid, raging and psychotic. Only the mildest forms can be left untreated without risking harm to either the patient's welfare, relationships and job, or to the well-being of those who are close to them (relatives, carers and mental health professionals). The milder forms may be accompanied by high levels of energy, productivity and creativity. But even these carry a risk of subsequently switching into a phase of depression and incapacity that might have been avoided by treatment of the preceding hypomania. Severe forms constitute a psychiatric emergency, demanding immediate control, including rapid tranquillisation with medication. Mania is one of the most insightless forms of mental disorder and for treatment to be very useful it must be not only effective but also acceptable to the patient, easy to use and not produce unpleasant side-effects (Reference Cookson, Stein and WilkinsonCookson, 2007).
Surveys of clinical practice have shown that antipsychotics are the most commonly used drugs for patients hospitalised with mania, whether in Britain, Scandinavia, other parts of Europe or North America. Classical (typical) antipsychotics produce unpleasant extrapyramidal side-effects such as akathisia, dystonia and parkinsonism, which (although partially preventable by anticholinergic medication) are resented by patients and limit their adherence to treatment. It is therefore very important to know whether the use of antipsychotics in mania is justified by evidence of efficacy and whether newer antipsychotics with fewer unpleasant acute side-effects are also effective in mania.
Mechanisms of antimanic actions of antipsychotics
It is thought that antipsychotics owe their anti-manic effects mainly to blockade dopamine receptors, but additionally to some extent to blockade of noradrenaline at receptors (as in the α1 case of haloperidol), and blockade of histamine at H1 receptors (causing sedation as in the case of chlorpromazine) (Reference Peroutka and SnyderPeroutka & Snyder, 1980; Reference CooksonCookson, 2001). Some atypical antipsychotics (e.g. olanzapine, quetiapine, risperidone) share all these actions as well as being potent blockers of serotonin receptors, but are selective for sub-types of dopamine receptors; others (amisulpride) block only sub-types of dopamine receptors. Blockade of serotonin (5-hydroxytryptamine, 5-HT) at 5-HT2A receptors is of unclear importance in mania. It cannot be assumed that drugs effective in schizophrenia will be effective in mania or vice versa.
Assessing the evidence
To prove that a drug is efficacious for a psychiatric condition, it is essential to show that it is superior to placebo, by conducting randomised double-blind placebo-controlled trials. The challenges of conducting such trials in mania have been met only in recent years, in the course of developing novel anticonvulsant and atypical antipsychotic treatments in trials since 1994. These trials are therefore providing answers to questions that have long remained unresolved about the treatment of mania. Analysis of the results of these trials requires attention not only to the statistical significance of differences in special rating scales, but also to the size of the effect, and to the generalisability of results derived from highly selected patients in clinical trials centres to patients with mania in routine practice. It is also important to consider how drop-outs from the studies may have biased the interpretation of results.
‘Acute’ or ‘rapid’ tranquillisation
Treatment of mania may begin with control of the agitated patient by ‘acute’ or ‘rapid’ tranquillisation (Reference Cookson, Akiskal and TohenCookson, 2006). This involves an antipsychotic or a benzodiazepine or a combination of these, which may have to be given intramuscularly. Two placebo-controlled trials have investigated the response of patients with mania to intramuscular medication. The first showed improvement within 20 min and greater improvement with the antipsychotic (olanzapine 10 mg) than with the benzodiazepine (lorazepam 2 mg) over 2 h (Reference Meehan, Zhang and DavidMeehan et al, 2001). The second showed improvement with both lorazepam (2 mg) and aripiprazole (9.75–15 mg) but a trend towards greater improvement with lorazepam (Reference Zimbroff, Marcus and ManosZimbroff et al, 2007). By contrast, intravenous valproate (20 mg/kg) was not associated with an improvement in mania within 2 h (Reference Phrolov, Applebaum and LevinePhrolov et al, 2004), suggesting a different mechanism of action.
Once calmed, most patients then require treatment over a period of 2–4 weeks with oral medication to achieve a more gradual further improvement.
Monotherapy comparisons with placebo in mania
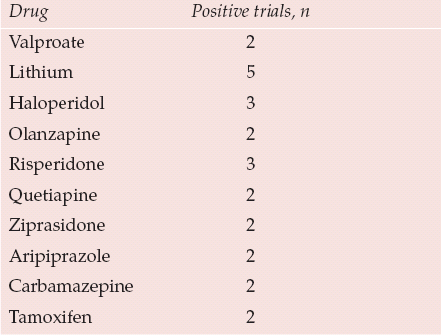
Table 1 lists the drugs that have been proved superior to placebo as monotherapy in such trials lasting 3–6 weeks. To grant a licence to market a drug for mania, most authorities, including the European Medicines Agency, require two trials performed at independent centres. Less well-proven, but probably effective, is clozapine (Reference Suppes, Webb and PaulSuppes et al, 1999). In addition, one can conclude from negative trials that certain drugs do not improve mania; these are topiramate, gabapentin and lamotrigine.
Table 1 Drugs shown to improve mania as monotherapy in randomised placebo-controlled trials
| Drug | Positive trials, n |
|---|---|
| Valproate | 2 |
| Lithium | 5 |
| Haloperidol | 3 |
| Olanzapine | 2 |
| Risperidone | 3 |
| Quetiapine | 2 |
| Ziprasidone | 2 |
| Aripiprazole | 2 |
| Carbamazepine | 2 |
| Tamoxifen | 2 |
Lithium, valproate or antipsychotics for mania
The first drug to be proved efficacious in such trials was valproate (Reference Pope, McElroy and KeckPope et al, 1991; Reference Bowden, Brugger and SwannBowden et al, 1994). Although Tables 2 and 3 show that the drug with most trials (not all published) proving efficacy is lithium, this drug is not usually sufficiently rapid in onset to be useful as monotherapy (Reference Bowden, Brugger and SwannBowden et al, 1994). The trials of carbamazepine (Reference Weisler, Kalali and KetterWeisler et al, 2004, Reference Weisler, Keck and Swann2005) and aripiprazole (Reference Sachs, Sanchez and MarcusSachs et al, 2006) are more recent. Tamoxifen, an anti-oestrogen, widely used for breast cancer, has antimanic properties that were first investigated because this drug shares with lithium and valproate the ability to block the intracellular second messenger system protein kinase-C, through which certain transmitters act (Reference Zarate, Singh and CarlsonZarate et al, 2007; Reference Yildiz, Guleryuz and AnkerstYildiz et al, 2008). Although haloperidol has been the favourite drug of clinicians for treating mania (Reference Chou, Zito and VitraiChou et al, 1996; Reference CooksonCookson, 2001), it is only in the course of comparative trials with risperidone (Reference Smulevich, Khanna and EerdekensSmulevich et al, 2005) and quetiapine (Reference McIntyre, Brecher and PaulssonMcIntyre et al, 2005), and more recently aripiprazole, that haloperidol has been proved conclusively to be efficacious. Apart from haloperidol, most evidence for efficacy in mania now concerns the atypical antipsychotics olanzapine, risperidone, quetiapine, ziprasidone and aripiprazole. Questions arise about the relative efficacy of these drugs compared with either haloperidol or valproate, and whether they should be used initially as monotherapy or combined with valproate or lithium. Also, since treatment often needs to commence with rapid tranquillisation, and only three atypicals can be given intramuscularly (recently olanzapine and in some countries ziprasidone and aripiprazole), haloperidol remains widely used despite its propensity to cause unpleasant extrapyramidal side-effects.
Table 2 Monotherapy with atypical antipsychotics in mania: numbers needed to treat (NNTs) in placebo-controlled parallel-group randomised trials

| Drop-out rate, % | |||||||
|---|---|---|---|---|---|---|---|
| Drug, mean daily dose and sample size | Duration (study) 1 | Criterion of improvement | Inefficacy | Adverse events | Response, % | Difference from placebo | NNT (95% CI) |
| Olanzapine, 14.9 mg: n=70 | 3 weeks (Reference Tohen, Sanger and McElroyTohen, 1999) | 50% reduction YMRS | 29 | 0 | 49 | 25 | 4 (3–10) |
| Placebo: n=64 | 48 | 3 | 24 | ||||
| Olanzapine, 16.4 mg: n=55 | 4 weeks (Reference Tohen, Jacobs and GrundyTohen, 2000) | 50% reduction YMRS | 34 | 4 | 65 | 22 | 5 (3–23) |
| Placebo: n=60 | 56 | 2 | 43 | ||||
| Risperidone, 4.1 mg: n=134 | 3 weeks (Reference Hirschfeld, Keck and KarcherHirschfeld, 2004) | 50% reduction YMRS | 36 | 8 | 43 | 19 | 6 (4–13) |
| Placebo: n=125 | 52 | 6 | 24 | ||||
| Risperidone, 5.6 mg: n=146 | 3 weeks (Reference Khanna, Vieta and LyonsKhanna, 2005) | 50% reduction YMRS | 10 | 0.7 | 73 | 37 | 3 (2–4) |
| Placebo: n=144 | 25 | 4.2 | 36 | ||||
| Risperidone: | |||||||
| 4.2 mg: n=154 | 3 weeks (Reference Smulevich, Khanna and EerdekensSmulevich, 2005) | 50% reduction YMRS | 7 | 4 | 48 | 15 | 7 (4–26) |
| Placebo: n=140 | 3 weeks, extended to 12 (Reference McIntyre, Brecher and PaulssonMcIntyre, 2005) | 50% reduction YMRS | 10 | 5 | 33 | 14 | 8 (4–37) |
| Haloperidol, 8 mg: n=144 | 7 | 3 | 47 | ||||
| Quetiapine, 560 mg: n=102 | 41 | 5 | 42 | 7 | NSD | ||
| Placebo: n=101 | 52 | 6 | 35 | 20 | 5 (3–16) | ||
| Haloperidol, 5.2 mg: n=99 | 35 | 10 | 55 | ||||
| Quetiapine, 586 mg: n=107 | 3 weeks, extended to 12 (Reference Bowden, Grunze and MullenBowden, 2005) | 50% reduction YMRS | 26.2 | 6.5 | 53.3 | 25.9 | 4 (3–8) |
| Placebo: n=95 | 59.8 | 4.1 | 27.4 | 25.9 | 4 (3–10) | ||
| Lithium: n=98 | 25.5 | 6.1 | 53.3 | ||||
| Aripiprazole, 27.7 mg: n=136 | 3 weeks (Reference Sachs, Sanchez and MarcusSachs, 2006) | 50% reduction YMRS | 9 | 9 | 53 | 21 | 5 (4–11) |
| Placebo: n=132 | 21 | 7 | 32 | ||||
| Aripiprazole, 27.9 mg: n=125 | 3 weeks (Reference Keck, Marcus and TourkodimitrisKeck, 2003a ) | 50% reduction YMRS | 47 | 11 | 40 | 21 | 5 (4–11) |
| Placebo: n=123 | 69 | 10 | 19 | ||||
| Ziprasidone, 80–160 mg: n=131 | 3 weeks (Reference Keck, Versiani and PotkinKeck, 2003b ) | 50% reduction | |||||
| MRS | 40 | 6.4 | 50 | 15 | 7 (4–153) | ||
| Placebo: n=66 | 51.4 | 4.7 | 35 | ||||
| Ziprasidone, 80–160 mg: n=139 | 3 weeks (Reference Potkin, Keck and SegalPotkin, 2005) | 50% reduction MRS | 33 | 5.8 | 46 | 16.8 | 6 (4–33) |
| Placebo: n=66 | 44 | 1.5 | 29.2 | ||||
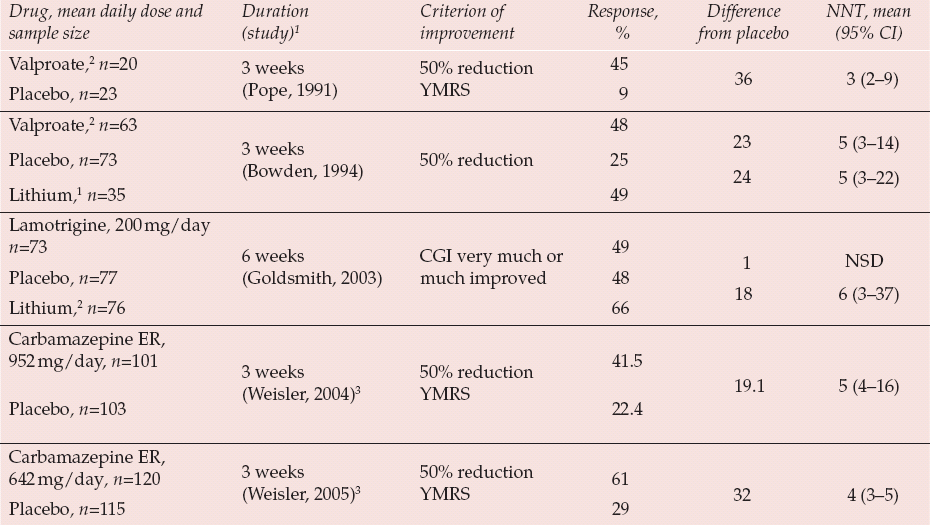
Table 3 Monotherapy with other antimanic drugs in mania: numbers needed to treat (NNTs) in placebo-controlled parallel-group randomised trials
| Drug, mean daily dose and sample size | Duration (study) 1 | Criterion of improvement | Response, % | Difference from placebo | NNT, mean (95% CI) |
|---|---|---|---|---|---|
| Valproate,2 n=20 | 3 weeks (Reference Pope, McElroy and KeckPope, 1991) | 50% reduction YMRS | 45 | 36 | 3 (2–9) |
| Placebo, n=23 | 9 | ||||
| Valproate,2 n=63 | 3 weeks (Reference Bowden, Brugger and SwannBowden, 1994) | 48 | 23 | 5 (3–14) | |
| Placebo, n=73 | 50% reduction | 25 | 24 | 5 (3–22) | |
| Lithium,1 n=35 | 49 | ||||
| Lamotrigine, 200 mg/day | |||||
| n=73 | 6 weeks (Reference Goldsmith, Wagstaff and IbbotsonGoldsmith, 2003) | CGI very much or much improved | 49 | 1 | NSD |
| Placebo, n=77 | 48 | 18 | 6 (3–37) | ||
| Lithium,2 n=76 | 66 | ||||
| Carbamazepine ER, 952 mg/day, n=101 | 3 weeks (Reference Weisler, Kalali and KetterWeisler, 2004)3 | 50% reduction YMRS | 41.5 | 19.1 | 5 (4–16) |
| 22.4 | |||||
| Placebo, n=103 | |||||
| Carbamazepine ER, 642 mg/day, n=120 | 3 weeks (Reference Weisler, Keck and SwannWeisler, 2005)3 | 50% reduction YMRS | 61 | 32 | 4 (3–5) |
| Placebo, n=115 | 29 |
Randomised placebo-controlled trials of atypical antipsychotics
The published trials are summarised in Table 2, using a ‘number needed to treat’ (NNT) analysis of size of effect. Number needed to treat is calculated by dividing the difference in response rate between active drug and placebo into 100 and correcting to the next highest integer. It represents the number of patients who must be treated for one patient to achieve the defined response as a result of the pharmacological effect of the drug. In this instance the response was usually a 50% reduction in score on the 11-item Young's Mania Rating Scale (YMRS; Reference Young, Biggs and ZieglerYoung et al, 1978) – a scale that clinicians may find helpful for monitoring the progress of in-patients with mania. The NNT thus provides a measure of the size of effect that can be expected of the drug in a clinical situation, and it may be more clinically meaningful than the statistically more precise measure known as ‘effect size’. For a drug to be useful monotherapy as a first-line treatment in a common and severe disorder such as mania, the NNT for 50% improvement in severity should be in the order of 2–4 (Reference Cookson, Taylor and KatonaCookson et al, 2002).
Most studies were of 3 or 4 weeks duration and there was a placebo response rate of 19–43%, reflecting the effects of a variety of possible non-specific factors such as hospitalisation, extra medication with benzodiazepines or chloral allowed during the first 10 days, and bias in the raters. Drop-out rates for inefficacy ranged from 10 to 69% on placebo, and from 7 to 47% on active drug. Drop-out rates for adverse events (including suspected side-effects) ranged from 1.5 to 10% on placebo, and from 0 to 11% on atypical antipsychotic; on haloperidol the drop-out rates for lack of efficacy were 7 and 35%, and for adverse events 3 and 10%. In some studies patients dropped out at their own request with no clear reason. Total drop-out rates ranged from 15 to 79% on placebo, from 11 to 58% on atypical antipsychotic, and were 10 and 45% on haloperidol and 32% on lithium (Table 2). When making comparisons between groups, loss of a participant through drop-out is usually dealt with by carrying forward the rating at the last observed time before drop-out. This method of analysis (‘last observation carried forward’ or LOCF) introduces a bias against any treatment, including placebo, that is associated with a high rate of drop-outs. The study with the lowest rate of drop-out on active treatment was the study of risperidone conducted in India (Reference Khanna, Vieta and LyonsKhanna et al, 2005). It is likely that all other studies underestimated the extent of antipsychotic efficacy.
Olanzapine
Olanzapine was the first of the atypical antipsychotics to be proved efficacious in mania (Reference Tohen, Sanger and McElroyTohen et al, 1999, Reference Tohen, Jacobs and Grundy2000). Patients with mixed mania were also included in these trials. The starting doses in the two studies were 10 mg and 15 mg, and the mean modal doses (modal dose for each patient, averaged for each therapy) were 14.9 and 16.4 mg/day. The most common side-effects were somnolence (22% more than placebo), dry mouth (15% more), dizziness (12% more), weakness (9% more) and weight gain (10% more). The problem of weight gain is more obvious in adolescents: a later study (Reference Tohen, Kryzhanovskaya and CarlsonTohen et al, 2007) recorded gain in 41.9% on olanzapine v. 1.9% on placebo.
Risperidone
In the trial of Reference Hirschfeld, Keck and KarcherHirschfeld et al (2004), risperidone was increased gradually over 4 days to a maximum of 6 mg/day. The NNT was 6 (95% CI 4–13). Side-effects on risperidone were somnolence (28% v. 7% on placebo) and hyperkinesias (parkinsonism) in 16% v. 5%.
The study by Reference Khanna, Vieta and LyonsKhanna et al (2005) was distinguished by relatively high YMRS scores on entry (mean score 37; about 30% higher than in most other studies of atypical antipsychotics in mania), and by a high rate of study completion on risperidone with low dropout rates, particularly for lack of efficacy. The mean modal dose was 5.4 mg/day. The rate of response on risperidone was highest in this study and the NNT was impressively low at 3. Extrapyramidal side-effects occurred in 35% of those on risperidone and 6% on placebo.
The study by Reference Smulevich, Khanna and EerdekensSmulevich et al (2005) had a slower dosing schedule, reaching a maximum of 6 mg/ day by day 5. It also had a haloperidol comparator group with a mean modal dose of 8 mg/day (see below). Both active drugs were effective compared with placebo from day 7. By day 21 the NNT for 50% improvement was 7 for risperidone and 8 for haloperidol. Side-effects on risperidone included extrapyramidal symptoms (17%, compared with 40% for haloperidol and 95% for placebo).
Quetiapine
The two trials of monotherapy excluded patients with mixed mania. Significant efficacy at 3 weeks was observed in one study and in the combined analysis (Reference Vieta, Mullen and BrecherVieta et al, 2005). Patients who responded to quetiapine were usually receiving 600 mg/day or more. The dose was increased towards this over 5 days and then to a maximum of 800 mg/day. The most common side-effects were somnolence, dry mouth, weight gain and dizziness.
Ziprasidone and aripiprazole
Aripiprazole is the most recent atypical antipsychotic to be licensed in the UK for the treatment of mania. Ziprasidone is not yet licensed for use in the UK. In the studies of aripiprazole and ziprasidone, additional sedation with lorazepam in the relatively high doses of up to 6 and 8 mg/day respectively was permitted for the first 4 days, implying a feared lack of rapid efficacy and control of agitation. For both these drugs the main advantages may be in long-term treatment when their side-effects on weight gain and metabolism are probably superior to other atypicals.
Placebo-controlled monotherapy trials of haloperidol in mania
Two monotherapy studies have included haloperidol as an active comparator (Reference McIntyre, Brecher and PaulssonMcIntyre et al, 2005; Reference Smulevich, Khanna and EerdekensSmulevich et al, 2005) (Table 2). In the former, the haloperidol dose started at 4 mg/day and was adjusted to 2–12 mg/day by day 5. The NNT for 50% improvement by day 21 was 8. This is far larger than one would expect with the most commonly used antimanic drug of the previous decade. This might be because the mean dose of haloperidol was only 8 mg/day, or because the patients in the trial were in some ways not typical of routine clinic patients and more resistant to treatment.
A comparator group on haloperidol (up to 8 mg/day) was also included in the study by Reference McIntyre, Brecher and PaulssonMcIntyre et al (2005). At 3 weeks the response rate on haloperidol, on a mean dose of only 5.2 mg/day, was 55% compared with 35% on placebo, giving an NNT of 5. Side-effects in the form of extra-pyramidal symptoms were much more common on haloperidol (59.6%) than on placebo (15.8%): for example, 33.3% of participants on haloperidol but only 5.9% on placebo experienced akathisia. Somnolence occurred more often with haloperidol (9.1%) than placebo (5%).
Patterns of symptom improvement: sedative, antipsychotic or antimanic?
It had been suggested that antipsychotics owe their effects in mania either to non-specific sedation (that is making the person drowsy or asleep), or to combating psychotic symptoms. However, this view fails to recognise that non-sedative dopamine-blocking drugs can improve mania (Reference Cookson, Silverstone and WellsCookson et al, 1981); these would now include aripiprazole and ziprasidone. In all studies of olanzapine and risperidone and in the combined analysis of quetiapine studies, the improvement in mania occurred in patients with or without psychotic symptoms. When individual items of the YMRS were analysed, drug treatment (with olanzapine, quetiapine and presumably the other antipsychotics) improved the whole range of symptoms (including elation, flight of ideas, grandiosity, sexual interest, irritability, aggression, general appearance and insight, as well as the items most sensitive to sedation: insomnia, overactivity and pressure of speech). These findings lead to the inevitable conclusion that the drugs are not just antipsychotic, but also antimanic. Interestingly, the symptom of loss of insight is one of the slowest to improve (Reference Tohen, Sanger and McElroyTohen et al, 1999). This corresponds to the clinical situation in which a patient treated with antipsychotics for severe mania has improved sufficiently within 3 weeks to appeal successfully to a mental health review tribunal, but still lacks insight into the illness and the benefits of, and need for, treatment.
Depression in mania
Depressive symptoms are very common during mania, and if amounting to a major depressive syndrome the condition is classified as mixed mania in DSM–IV (American Psychiatric Association, 1994). However, at least 12 forms of bipolar mixed states have been described and are likely to respond differently to treatments (Reference Cookson, Ghalib, Marneros and GoodwinCookson & Ghalib, 2005). Some patients develop depressive syndromes after mania has improved (‘post-manic depression’), and this is described as a ‘switch’ into depression.
It has been suggested, but never proved, that classical antipsychotics may worsen or induce depression apart from their obvious extrapyramidal side-effects. For example, the use of perphenazine in mania without an anticholinergic drug has been associated with a high rate of development of depressive symptomatology, with accompanying signs of parkinsonism, particularly akinesia (Reference Zarate and TohenZarate & Tohen, 2004). Used in this way for schizophrenia, the older antipsychotics such as haloperidol are known to induce ‘akinetic depression’, which is best viewed as an extrapyramidal side-effect (Reference van Putten and Mayvan Putten & May, 1978).
In the trials of atypical antipsychotics in mania, changes in symptoms of depression have usually been monitored. Thus, several antipsychotics have been shown to improve depressive symptoms alongside the improvement in mania; these include olanzapine (Reference Tohen, Sanger and McElroyTohen et al, 1999, Reference Tohen, Jacobs and Grundy2000), risperidone (Reference Khanna, Vieta and LyonsKhanna et al, 2005), quetiapine and aripiprazole. For example, in one study (Reference Smulevich, Khanna and EerdekensSmulevich et al, 2005), depression scores (on the Montgomery–Åsberg Depression Rating Scale, MADRS) fell more on risperidone than on placebo from week 1, and on haloperidol only from week 2.
Likewise, on both quetiapine and haloperidol, depression scores improved by day 21 more than on placebo (Reference McIntyre, Brecher and PaulssonMcIntyre et al, 2005). On the other hand, the switch rates into depression over 12 weeks were similar for haloperidol (8.1%) and placebo (8.9%), and tended to be lower for quetiapine (2.9%).
In patients with mixed mania, both depression (MADRS) and mania scores improved with treatment on olanzapine (v. placebo) used either as monotherapy (Reference Baker, Tohen and FawcettBaker et al, 2003) or as an adjunct to lithium or valproate (Reference Baker, Brown and AkiskalBaker et al, 2004).
Comparative RCTs of anti-psychotics in mania without placebo: haloperidol v. atypicals
In a comparative trial in mania, in which additional lorazepam was permitted, risperidone showed similar efficacy to haloperidol or lithium (Reference Segal, Berk and BrookSegal et al, 1998).
In the largest randomised comparative study of haloperidol (Reference Tohen, Goldberg and Gonzalez-PintoTohen et al, 2003), it was compared with olanzapine over 6 and 12 weeks. Among patients on haloperidol (up to 15 mg/day, at week 6 mean dose 7 mg/day), the proportion responding (50% reduction in YMRS score) by 6 weeks was 74%. The proportion showing syndromal remission (according to DSM–IV) was 44%, a figure similar to that found on haloperidol in consecutive admissions for mania by Reference Rifkin, Doddi and KarajgiRifkin et al (1994).
In patients with low levels of depressive symptoms at commencement on haloperidol, a total of 16.8% switched into depression within 12 weeks. However, as there was no placebo group, it is not clear whether this represents the natural history of the patients' mood cycles, perhaps accelerated by effective treatment of mania, or some additional depressant effect of haloperidol. The switch rate among patients on olanzapine was non-significantly lower at 12 weeks (9.4%), although the switch to depression occurred significantly sooner with haloperidol (Reference Tohen, Goldberg and Gonzalez-PintoTohen et al, 2003).
Thus, both olanzapine and quetiapine tended to produce a lower rate of switching into depression than did haloperidol. Both drugs (in the doses used) seemed also to lead to slower improvement in mania than did haloperidol. There is also the problem that haloperidol is prescribed in double-blind trials without a prophylactic anticholinergic drug, and is therefore liable to induce ‘akinetic depression’, as described by Reference van Putten and Mayvan Putten & May (1978) in schizophrenia. When this occurs, some studies allow that an anticholinergic be added; others discontinue the patient from the trial.
These studies confirm the efficacy of haloperidol in reducing the symptoms of mania. None of the newer drugs has been shown to be more effective in this regard. Most (risperidone is the exception) appear to be less effective than haloperidol (Reference Scherk, Pajonk and LeuchtScherk et al, 2007). However, the doses used in the trials for both the atypical antipsychotics and for haloperidol may be less than sufficient to produce optimal improvement. The clear superiority of the newer antipsychotics is that they produce far fewer extrapyramidal side-effects than haloperidol. For example, the very unpleasant side-effect of akathisia was reported by 30% of patients on haloperidol and 6% on olanzapine.
Trials of atypical antipsychotics v. valproate in mania
Olanzapine (Reference Tohen, Baker and AltshulerTohen et al, 2002 , Reference Tohen, Goldberg and Gonzalez-Pinto2003; Reference Zajecka, Weisler and SachsZajecka et al, 2002), quetiapine (Reference Del Bello, Kowatch and AlderDel Bello et al, 2006) and haloperidol (Reference McElroy, Keck and StantonMcElroy et al, 1996) have been compared directly with valproate. The improvement was slightly faster and slightly greater with olanzapine (average doses 17.4 and 14.7 mg/day) than with semisodium valproate (1401 and 2115 mg/day). Interestingly, this superiority of olanzapine over valproate was seen only in patients with non-psychotic mania (Reference Tohen, Baker and AltshulerTohen et al, 2002 ). Olanzapine and valproate seemed equally effective in psychotic mania as they did in mixed mania.
A comparison of quetiapine with valproate in adolescents with mania also showed superiority of the antipsychotic (Reference Del Bello, Kowatch and AlderDel Bello et al, 2006).
Apart from speed of action, which is greater with antipsychotics, there are differences in side-effects. Olanzapine produced more somnolence (39% v. 21%, and 47% v. 29%), dry mouth (34% v. 6%), running nose (14% v. 3%), oedema (14% v. 0%), increased appetite (12% v. 2%) and weight gain (12% v. 7.9%, and 25% v. 10%), whereas valproate produced more gastrointestinal disturbance with nausea (29% v. 10%). Other side-effects of valproate and olanzapine occur but are too rare to have been detected in these trials.
The study of psychotic mania by Reference McElroy, Keck and StantonMcElroy et al (1996) indicated that if a sufficiently large dose of valproate (as semisodium valproate 20 mg/kg/day) is used from the start, a similar improvement occurs with valproate or haloperidol (0.2 mg/kg/day). However, the generalisability of this finding may be limited, since haloperidol did not show its usual rapid onset of effect. Furthermore, a second study of valproate loading by Reference Hirschfeld, Allen and McEvoyHirschfeld et al (1999) showed a delay of about 48 h in onset of the antimanic effect with valproate.
Monotherapy or combination treatment
Since lithium and valproate are thought to have mechanisms of action other than receptor blockade, and probably reduce dopamine release, it may be expected that combination of an antipsychotic with lithium or valproate will produce a greater antimanic effect. This has been confirmed in trials in which an antipsychotic or placebo was given in addition to lithium or valproate. The effect is clearest when patients had previously shown only partial response to the lithium or valproate. The antipsychotics for which this added benefit has been shown are olanzapine, risperidone, quetiapine and aripiprazole, but not ziprasidone (Reference Scherk, Pajonk and LeuchtScherk et al, 2007). The contrary situation in which valproate or placebo has been added to an antipsychotic that patients were already receiving has been reported in only one study (Reference Muller-Oerlinghausen, Retzow and HennMuller-Oerlinghausen et al, 2000).
Two earlier trials found carbamazepine together with a typical antipsychotic more effective than an antipsychotic alone (Reference Klein, Bental and LererKlein et al, 1984; Reference Moller, Kissling and RiehlMoller et al, 1989). However, more recent trials failed to find a benefit of adding risperidone or olanzapine to carbamazepine (Reference Yatham, Grossman and AugustynsYatham et al, 2003; Reference Tohen, Bowden and SmulevichTohen et al, 2008); in the latter trial a high dose of olanzapine was given to counter the increased metabolism due to hepatic enzyme induction on carbamazepine.
Conclusions
All the atypical antipsychotics that have been studied in rigorous randomised controlled trials in mania have been shown to be superior to placebo. Of the commonly used drugs, only amisulpride has not been studied in this way.
The clinical trials of atypical antipsychotics in mania, sponsored by the pharmaceutical manufacturers, have answered many important questions about bipolar disorder that had been unresolved for 50 years. In particular, they have shown that antipsychotics generally have specific antimanic properties that are independent of sedation or psychosis. The speed of action and size of effect of antipsychotics makes them especially useful for control of emergent (hypomanic) symptoms and for acute tranquillisation in mania. None of the atypicals is more effective than haloperidol in reducing manic symptoms, but all produce fewer extrapyramidal side-effects than haloperidol and are therefore more acceptable to patients. In addition, some atypicals are associated with less post-manic depression, which can be another manifestation of extrapyramidal effects (akinetic depression). Most current guidelines for the treatment of bipolar disorder (e.g. Reference Cookson, Kasper and HirschfeldCookson, 2005; National Collaborating Centre for Mental Health, 2006) recommend an antipsychotic (preferably an atypical) either alone or as part of first-line treatment of mania.
Declaration of interest
J. C. has provided advice and lectures at meetings sponsored by the manufacturers of several atypical antipsychotics, including those mentioned in this article.
EMIs
-
1 Theme: antipsychotics
Options
-
a Haloperidol
-
b Risperidone
-
c Olanzapine
-
d Quetiapine
-
e Aripiprazole
-
f Ziprasidone
-
g Clozapine.
Choose the drug:
-
i which has not yet been proved superior to placebo as monotherapy for mania
-
ii which has failed to show superiority to placebo when combined with lithium or valproate for mania
-
iii which is the most commonly used for acute tranquillisation
-
iv which showed the lowest NNT for response in mania.
-
-
2 Theme: anticonvulsants and lithium
Options
-
a Lithium
-
b Valproate
-
c Lamotrigine
-
d Carbamazepine
-
e Topiramate
-
f Gabapentin.
Choose the drug:
-
i which, other than lithium and valproate, was efficacious as monotherapy for mania
-
ii with which olanzapine failed to show efficacy in mania when it was added in combination
-
iii which, other than lithium, has been proved efficacious in severe (psychotic) mania
-
iv which has the most randomised placebo-controlled trials showing efficacy in mania.
-
-
3 Theme: symptoms and syndromes
Options
-
a Depressive symptoms
-
b Post-manic depression
-
c Akinetic depression
-
d ‘Switch’ from mania to depression
-
e The whole range of manic symptoms
-
f Agitation and psychotic symptoms.
Choose the symptom or syndrome:
-
i which is rated on the YMRS
-
ii which in counteracted by anticholinergic medication
-
iii which is most common during an episode of mania
-
iv which respond most clearly to olanzapine or quetiapine.
-
EMI correct matchings

| 1 | 2 | 3 | |||
|---|---|---|---|---|---|
| i | g | i | d | i | e |
| ii | f | ii | d | ii | c |
| iii | a | iii | b | iii | a |
| iv | b | iv | a | iv | e |






eLetters
No eLetters have been published for this article.