Bipolar disorder is a disabling, largely episodic, recurrent illness associated with severe functional impairment, psychiatric and somatic comorbidity, and premature mortality from both suicide and medical illnesses (Reference Baldessarini and Salvatore R KhalsaBaldessarini 2010a,Reference Baldessarini, Vieta and Calabreseb; Reference Sanders and GoodwinSanders 2010). Lifetime prevalence of bipolar disorder in the general population, taking into account both type I (with mania) and type II (with hypomania) disorder, is at least 1–2% (Reference Merikangas, Jin and HeMerikangas 2011), and up to 10% if broad diagnostic criteria include major depression with subthreshold hypomania (Reference Zimmermann, Brückl and NoconZimmermann 2009).
Depressive, dysthymic and mixed (dysphoric/agitated) states contribute to the total illness burden in bipolar disorder. These morbidity factors are strongly associated with, and predicted by, similar first lifetime episodes (Reference Baldessarini and Salvatore R KhalsaBaldessarini 2010a, Reference Baldessarini, Undurraga and Vázquez2012). In several longitudinal studies of the treatment of bipolar disorder by community standards, the mean proportion of weeks in morbid states was 68% and three-quarters of that unresolved morbidity is accounted for by depressive illness (Reference Baldessarini and Salvatore R KhalsaBaldessarini 2010a; Reference Tondo, Baldessarini and VázquezTondo 2013). Depressive components of bipolar disorder are associated not only with a high proportion of unresolved (treatment-resistant) morbidity, but also with psychiatric and medical comorbidity, disability and mortality from suicide in young patients and from co-occurring medical disorders in older patients – all resulting in very high levels of clinical and economic burden for patients, families and society (Reference Ösby, Brandt and CorreiaÖsby 2001; Reference Tondo, Lepri and BaldessariniTondo 2007; Reference Crump, Sundquist and WinklebyCrump 2013).
Despite the high prevalence and major clinical, public health and economic significance of depression in bipolar disorder, few treatments have proved to be highly and consistently effective in acute episodes, and there is even less evidence of means of providing substantial long-term protection from recurrent episodes. In particular, there is considerable controversy about the value and risks of antidepressant drugs in treating bipolar depression (Reference Pacchiarotti, Bond and BaldessariniPacchiarotti 2013; Reference Vázquez, Tondo and UndurragaVázquez 2013). In turn, lack of highly effective treatments encourages widespread empirical trials of combination therapies (polytherapy) that are largely untested for effectiveness and safety.
It is likely that the paucity of therapeutic studies for bipolar depression reflects a broadly accepted view that major depression is similar in its clinical characteristics as well as its responses to treatment in patients with bipolar as well as unipolar mood disorder (Reference BaldessariniBaldessarini 2013a). Instead, there is considerable evidence that bipolar and unipolar mood disorders differ in many ways, including family history, gender distribution, age at onset, long-term diagnostic stability, episode duration, recurrence rates and response to treatment (Reference Baldessarini, Faedda and OffidaniBaldessarini 2013b).
These considerations indicate that bipolar depression remains a leading clinical problem and one of the most critical unsolved challenges for contemporary psychiatric therapeutics (Reference Baldessarini, Vieta and CalabreseBaldessarini 2010b, Reference Baldessarini2013a). We briefly discuss here the status of research evidence on drugs available to treat the depressive components of bipolar disorder, relying heavily on a series of recently reported meta-analyses (Reference Undurraga and BaldessariniUndurraga 2012a; Reference Tondo, Baldessarini and VázquezTondo 2013; Reference Vázquez, Tondo and UndurragaVázquez 2013; Reference Selle, Schalkwijk and VázquezSelle 2014). We focus on antidepressants as well as antimanic anti convulsants and lithium (as putative mood-stabilising agents), and on emerging evidence for modern, second-generation antipsychotics.
Summaries are provided of efficacy estimates (response rate ratio (RR), usually based on ≥50% reduction in symptom ratings) after randomisation to the drugs discussed v. placebo in controlled, randomised, monotherapy trials lasting an average of 8 weeks.
Antidepressants
Bipolar depression
The apparent ease and relative safety of treatment of major depressive episodes with antidepressants, combined with the strong wish of patients and their clinicians to minimise or avoid depression, has made antidepressants the leading treatment (Reference Baldessarini, Henk and SklarBaldessarini 2008). As noted, it is likely that this circumstance reflects uncritical acceptance of the broad concept of major depression (Reference BaldessariniBaldessarini 2013a). The tendency to view all forms of depression as similarly responsive to particular treatments has probably discouraged pharmaceutical manufacturers from conducting additional therapeutic trials specifically designed to test for treatment effects in depressive, dysthymic and mixed states of bipolar disorder, and to differentiate responses in bipolar type I and II disorder. An important contributing factor is that a known diagnosis of bipolar disorder has been an exclusion criterion from most controlled trials of antidepressants. This exclusion may be driven by a desire to avoid inducing mood switching or other potentially dangerous adverse behavioural effects, and associated potential legal liability, whether such concerns are warranted or not. The outcome is that antidepressants are both the most commonly employed treatment for bipolar disorder (Reference Baldessarini, Henk and SklarBaldessarini 2008) and one of the most controversial (Reference Pacchiarotti, Bond and BaldessariniPacchiarotti 2013; Reference Vázquez, Tondo and UndurragaVázquez 2013). Many experts call for caution in the use of antidepressants, discouraging their use in monotherapy and, if needed, recommend prescribing only in combination with mood-stabilising agents or second-generation antipsychotics (Reference Pacchiarotti, Bond and BaldessariniPacchiarotti 2013).
Current expert opinions about the value and potential risks of antidepressants to treat bipolar depression are greatly constrained by the limited and inconsistent research-based information that is available despite more than half a century of research and clinical use (Reference Gijsman, Geddes, Rendell and NolenGjisman 2004; Reference Vázquez, Tondo and BaldessariniVázquez 2011, Reference Vázquez, Tondo and Undurraga2013; Reference Sidor and MacQueenSidor 2012; Reference BaldessariniBaldessarini 2013a; Reference Pacchiarotti, Bond and BaldessariniPacchiarotti 2013; Reference Yatham, Kennedy and ParikhYatham 2013; Reference Tondo, Baldessarini and VázquezTondo 2014). Therapeutics research is very limited with regard to acute bipolar depression, and nearly lacking with respect to potential long-term benefits and risks (Reference Ghaemi, Wingo and FilkowskiGhaemi 2008; Reference Pacchiarotti, Bond and BaldessariniPacchiarotti 2013). Moreover, treatments for important features of bipolar disorder that are depressive (dysthymia, dysphoric mixed-states) but that do not represent acute major depressive episodes are especially poorly studied. Working out optimal clinical procedures to manage depressive components of bipolar disorder requires major attention, with considerable urgency with respect to bipolar type II disorder, in which depression is the main clinical concern and in which suicide rates are about as high as in bipolar type I disorder (Reference Tondo, Lepri and BaldessariniTondo 2007).
Efficacy in depressive episodes
Well-designed, controlled monotherapy trials focusing on the efficacy of antidepressants for acute bipolar depression are surprisingly rare, variable in size and quality, and have yielded notably inconsistent findings (Reference Vázquez, Tondo and BaldessariniVázquez 2011, Reference Vázquez, Tondo and Undurraga2013; Reference Sidor and MacQueenSidor 2012; Reference BaldessariniBaldessarini 2013a; Reference Pacchiarotti, Bond and BaldessariniPacchiarotti 2013; Reference Yatham, Kennedy and ParikhYatham 2013; Reference Tondo, Baldessarini and VázquezTondo 2014).
Two large trials are often cited as providing compelling support for the lack of efficacy of antidepressant treatment in acute bipolar depression. They call for comment owing to designs that may limit interpretation of their findings. The problem is that third arms of trials often represent secondary interests and are often smaller than the main arms (usually test drug of commercial interest v. placebo).
The first study, not a monotherapy trial, found no additional achievement of sustained remission of depressive symptoms by the addition of an antidepressant (paroxetine or bupropion) to a mood stabiliser (Reference Sachs, Nierenberg and CalabreseSachs 2007). The second study randomised a small sample of patients with bipolar depression to paroxetine in an 8-week trial designed primarily to test the efficacy of quetiapine. Quetiapine (n = 492 patients) was statistically superior to placebo (n = 126 patients), but did not show a dose-dependent difference between 300 and 600 mg/day, whereas paroxetine (n = 122 patients) was not superior to placebo (Reference McElroy, Weisler and ChangMcElroy 2010). In contrast, a recent meta-analysis including these and other relevant trials gives some support to the possible efficacy of antidepressants (Reference Vázquez, Tondo and UndurragaVázquez 2013) (Box 1).
BOX 1 Efficacy of antidepressants for bipolar depression
A recent meta-analysis of ten randomised, placebo-controlled, monotherapy trials of antidepressants in acute bipolar depression (Reference Vázquez, Tondo and UndurragaVázquez 2013) found a highly significant pooled difference favouring antidepressant treatment (bipolar response rate ratio (RR) 1.43, 95% CI 1.11–1.84; z-score = 2.76, P = 0.006), with an estimated number needed to treat of 6.2 (95% CI 3.6–6.7); the addition of three recent trials to the meta-analysis supported the same conclusion (Reference Selle, Schalkwijk and VázquezSelle 2014). Also notably, the pooled antidepressant/placebo RR in bipolar depression was not less than that found in a comprehensive meta-analysis of 122 randomised controlled trials in unipolar major depression (unipolar RR = 1.42 (95% CI 1.38–1.48); Reference Undurraga and BaldessariniUndurraga 2012a) (Table 1). There also was no appreciable difference in responses between patients with bipolar and unipolar depression compared directly in the same trials (Reference Vázquez, Tondo and BaldessariniVázquez 2011).
A recent comparison of clinical responses in large samples of patients with bipolar type I/ type II depression or recurrent unipolar major depressive disorder found only minor differences in rates of response or remission by diagnostic type, with low risks of mood switching – provided that patients with evidence of agitation or even subclinical hypomania at baseline were excluded (Reference Tondo, Baldessarini and VázquezTondo 2013). Perhaps the impression that antidepressants are less effective in bipolar than unipolar depression to some extent reflects adverse outcomes associated with increased agitation, anger or dysphoria interpreted as worsening of depression (Reference Tondo, Baldessarini and VázquezTondo 2014). Such reactions may also increase the considerable risk of suicidal behaviour in people with bipolar disorder, although specific effects of antidepressant treatment on suicide risk in this population remain uncertain and require further study.
In contrast to this incomplete state of research-based knowledge, it is widely assumed clinically that antidepressants may be appropriate for some patients with bipolar disorder, and especially those with type II disorder. Selection of patients as candidates for a trial of an antidepressant may usefully be guided by previous beneficial and well-tolerated responses to antidepressants, a less severe or less rapid illness course, few previous depressive episodes preceding mania, and lack of current agitation (Reference Pacchiarotti, Bond and BaldessariniPacchiarotti 2013).
Mood switching
There has been particular concern about pathological activation of mood and behaviour during treatment with antidepressants, stimulants or other mood-elevating drugs (e.g. corticosteroids) – either as a risk specific to having overt or potential bipolar disorder or as a psychotoxic effect. Risks specific to patients with bipolar type I disorder include potentially severe reactions involving mania, psychosis, aggression or irresponsible risk-taking, with associated liability concerns for clinicians and investigators. However, the frequency and severity of such reactions as well as the effects of measures that might limit such risks (e.g. co-treatment with a mood-stabilising or antimanic agent) remain unresolved matters requiring further research.
In a comprehensive review, we found that risk of spontaneous mania without antidepressants was high (averaging 13.8%), but that additional risk associated with antidepressant treatment increased risk by only 1.5% (Reference Tondo, Vázquez and BaldessariniTondo 2010) (Box 2). In addition, evidence from randomised trials indicates that antidepressants vary significantly in their association with mood switching, which appears to be especially high with tricyclic antidepressants and the serotonin-noradrenaline reuptake inhibitor (SNRI) venlafaxine (Reference Tondo, Vázquez and BaldessariniTondo 2010).
BOX 2 Risk of mood switching
A comprehensive review of studies comparing spontaneous and antidepressant-associated mood switching into mania or hypomania in patients with bipolar type I or II depression found average rates of 13.8% v. 15.3%, without v. with an antidepressant, indicating a small effect of antidepressants but a high risk of spontaneous switching (Reference Tondo, Vázquez and BaldessariniTondo 2010). Another review (Reference Sidor and MacQueenSidor 2012) found a pooled rate of switching of 8.0% in short-term, controlled treatment trials for bipolar depression, with a trivially greater risk with antidepressants (response rate ratio RR = 1.03, 95% CI 0.70–1.52). More recently, a review found an 8-week risk of 4.7% (95% CI 1.8–7.5) with placebo, and somewhat lower average risk of 3.7% (95% CI 2.1–5.3) after randomisation to a mood stabiliser or antipsychotic in 15 trials for bipolar depression (Reference Selle, Schalkwijk and VázquezSelle 2014). These observations suggest that concerns about risks of mood switching specific to antidepressants in patients with bipolar disorder may be greater than is warranted, although risks of spontaneous mania and hypomania, especially without a mood-stabilising agent in place, are substantial in patients with bipolar depression.
Available information about the epidemiology of mood-switching rates in patients is surprisingly limited. It does not include clear quantitative and qualitative distinctions between bipolar type I and type II disorder. In addition, there is a failure to report exposure times, estimates of risk-per-time or to define the time course of mood shifts with v. without antidepressant treatment. Lack of information about time-at-risk makes it difficult to distinguish between spontaneous and antidepressant-associated switch risk. Importantly, too, prospective, randomised comparisons of switching rates with v. without ongoing mood-stabilising or antipsychotic treatment are lacking. Meta-analysis even suggests, paradoxically, that risk of mood switching may be greater with a mood stabiliser included than with an antidepressant alone (Reference Tondo, Vázquez and BaldessariniTondo 2010). However, such findings arise almost entirely from observations involving clinically selected treatments that surely involve confounding by indication, or use of mood stabilisers where risks are considered to be elevated based on history or current behaviour (Reference Tondo, Vázquez and BaldessariniTondo 2010). Clinical and liability concerns about risk of mood switching (even if mainly spontaneous) contribute to the routine exclusion of patients with known bipolar disorder from treatment trials of agents with mood-elevating potential (Reference Undurraga, Baldessarini and ValentiUndurraga 2012b).
Despite this insufficient state of research, there is an evident clinical consensus that antidepressants should be used in patients with bipolar disorder only cautiously, briefly, in limited doses with short-acting agents, and in association with an effective mood-stabilising regimen, while monitoring closely for signs of emerging hypomania. We recommend that antidepressants not be used for bipolar depression if there is a history of mood switching during antidepressant treatment or if there is clinical evidence of current agitation or hypomanic symptoms (Reference Pacchiarotti, Bond and BaldessariniPacchiarotti 2013). Tricyclic antidepressants and SNRIs should be used with extra caution. Such practices are plausible but require prospective testing to support sound clinical practice (Reference Baldessarini, Faedda and OffidaniBaldessarini 2013b).
Long-term use of antidepressants
The potential value and risks of long-term use of antidepressants in both patients with bipolar type I and II disorder, with the intent of limiting the risk of future depressive episodes, remains poorly studied. Again, the lack of research support appears to have little impact on empirical trials of such treatment in clinical practice (Reference Baldessarini, Henk and SklarBaldessarini 2008, Reference Baldessarini2013a; Reference Ghaemi, Wingo and FilkowskiGhaemi 2008). Moreover, the value of long-term antidepressant treatment beyond the initial months of recovery from an index episode, even of unipolar major depression, remains uncertain, with a high risk of findings being confounded by clinically adverse effects of discontinuing treatment rather than due to lack of treatment (Reference Baldessarini, Tondo and GhianiBaldessarini 2010c, Reference Baldessarini, Faedda and Offidani2013b). However, long-term use of antidepressants along with mood stabilisers may be appropriate in response to relapses after discontinuing an antidepressant, especially if discontinuation is gradual (Reference Pacchiarotti, Bond and BaldessariniPacchiarotti 2013). It is not possible to recommend a specific duration for safe and effective continuation of antidepressant treatment. Generally, such treatment is managed in accordance with clinical response, and ideally with gradual removal of the antidepressant once the depression has remitted.
Two randomised controlled trials of continuation of antidepressants included patients who already had a favourable short-term response to the same treatments (Reference Leverich, Altshuler and FryeLeverich 2006; Reference Ghaemi, Ostacher and El-MallakhGhaemi 2010). Both studies suggest that a minority of patients may experience some delay or reduced frequency of depressive recurrences, with an even larger risk of mood switching. Moreover, rapid-cycling patients had an increased number of recurrent episodes with an antidepressant included in their treatment regimen, suggesting cycle acceleration (Reference Ghaemi, Ostacher and El-MallakhGhaemi 2010; Reference Pacchiarotti, Bond and BaldessariniPacchiarotti 2013).
Anticonvulsants
In recent decades, anticonvulsants have been widely used to treat bipolar disorder, based mainly on evidence of short-term antimanic effects (e.g. carbamazepine, valproate) and long-term protective effects of lamotrigine to limit risk of recurrent bipolar depressive episodes. However, the evidence of long-term, prophylactic effectiveness of anticonvulsants is less robust (Reference Geddes and MiklowitzGeddes 2013). Their use has also been encouraged to avoid the complexities of managing bipolar disorder with lithium (Reference BaldessariniBaldessarini 2013a). Other anticonvulsants (e.g. gabapentin, levetiracetam, oxcarbazepine, pregabalin, topiramate) are either inadequately evaluated or have been found to be ineffective (Reference BaldessariniBaldessarini 2013a; Reference Geddes and MiklowitzGeddes 2013; Reference Reinares, Rosa and FrancaReinares 2013;). Despite the widespread use of anticonvulsants to treat mania and efforts to afford long-term protective effects in patients, evidence concerning the value and risks of this class of drugs to treat acute bipolar depression is limited, and evidence concerning long-term effects is even more limited (Reference Reinares, Rosa and FrancaReinares 2013).
Four small trials involving, in total, fewer than 100 patients suggest some value of divalproex as monotherapy for acute bipolar depression (Reference Muzina, Gao and KempMuzina 2011) (Table 1, Fig. 1). Lamotrigine may have some effect in acute bipolar depression, based on pooling inconsistent data across individual trials, some of which failed to show superiority over placebo (Table 1, Fig. 1). However, lamotrigine is approved by the US Food and Drug Administration (FDA) only for long-term prophylaxis in borderline personality disorder, with much greater effectiveness against recurrent bipolar depression than mania (Reference Frye, Ha and KanbaFrye 2011). Moreover, the need for slow increments of doses to avoid dermatological reactions makes lamotrigine somewhat impractical for use in acute phases of bipolar disorder. Evidence concerning carbamazepine is very limited and controlled trials for other anticonvulsants are lacking (Reference Reinares, Rosa and FrancaReinares 2013; Reference Selle, Schalkwijk and VázquezSelle 2014).
TABLE 1 Treatments for acute depression in bipolar or unipolar major depressive episodes: meta-analyses of placebo-controlled trials

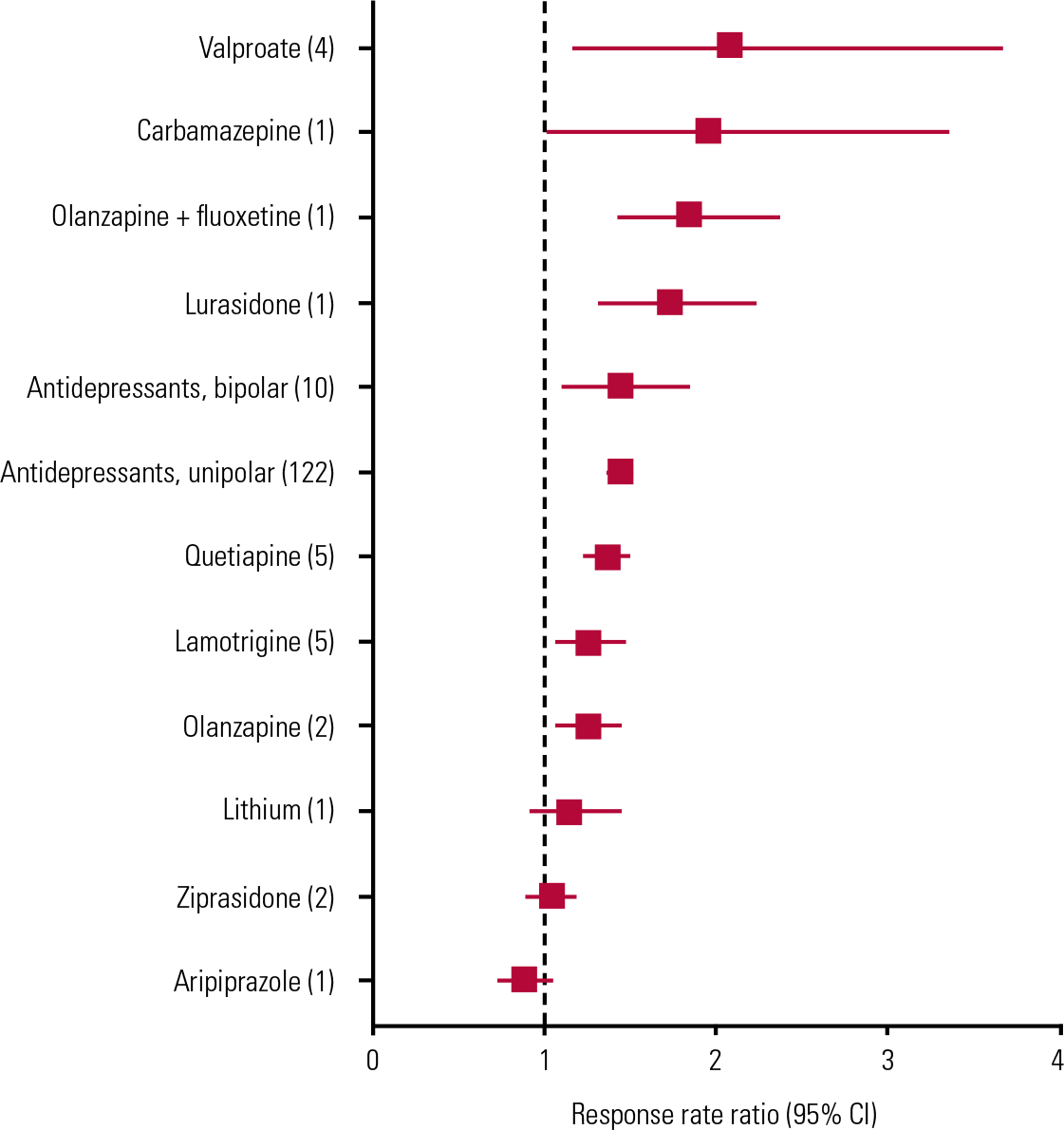
FIG 1 Forest plot of results of random effects meta-analyses of placebo-controlled monotherapy trials of treatments for acute bipolar depression. Based on data summarised and references in Table 1. Numbers of reported trials for each treatment are shown in parentheses. Data are pooled drug–placebo response rate ratios (RRs, with 95% CIs) from 34 randomised placebo-controlled trials of antidepressants (10 trials), mood-stabilising anticonvulsants (10 trials), second-generation antipsychotics (13 trials) or lithium (1 trial) in acute bipolar depression. Horizontal bars are computed CIs; vertical dashed line is the null value of RR = 1.00. Aripiprazole, lithium and ziprasidone did not separate statistically from placebo, and effects of other, apparently effective, drugs are not clearly differentiated owing to overlapping CIs, thus precluding ranking by apparent efficacy.
Lithium
There is remarkably little information concerning the effects of lithium in acute bipolar depression, despite its use as a fundamental treatment for bipolar disorder for more than six decades and being recommended as first-line treatment in some guidelines (Reference Yatham, Kennedy and ParikhYatham 2013). Its explicit use for bipolar depression is based on a single modern controlled trial, in which it was included as the third arm of a study designed primarily to evaluate quetiapine (Reference Young, McElroy and BauerYoung 2010; Table 1, Fig. 1). However, several small trials from the early 1970s, mostly based on crossover designs (usually lithium to placebo), suggest rates of response averaging about 73% in 100 patients with bipolar disorder and depression (Reference Zornberg and PopeZornberg 1993).
Nevertheless, lithium may have some long-term benefits against recurrent bipolar depression as well as its more prominent effects against mania and hypomania (Reference Baldessarini and Salvatore R KhalsaBaldessarini 2010a). Moreover, lithium appears to substantially reduce the risk of suicide in patients with bipolar disorder (Reference Baldessarini, Tondo and DavisBaldessarini 2006; Reference Cipriani, Hawton and StocktonCipriani 2013). Some experts, based mainly on research in unipolar major depression, also recommend lithium to augment the effects of other treatments (Reference Yatham, Kennedy and ParikhYatham 2013).
Second-generation antipsychotics
Antipsychotic drugs, including olanzapine combined with fluoxetine, as well as quetiapine and lurasidone, are currently the only medicines with FDA approval for the short-term treatment of acute major depressive episodes in bipolar disorder (Reference BaldessariniBaldessarini 2013a; Reference Selle, Schalkwijk and VázquezSelle 2014). Lurasidone received approval in June 2013, so the evidence base is as yet small (Reference Loebel, Cucchiaro and SilvaLoebel 2014). For the other drugs, responses are modest in adults, and quetiapine may not be effective in adolescent bipolar depression (Reference DelBello, Chang and WelgeDeBello 2009). Trials of quetiapine found no dose-dependent differences in efficacy (with 300 v. 600 mg/day), and only the lower dose is explicitly FDA-approved. The combination of olanzapine and fluoxetine produced superior benefits to those associated with lamotrigine in a rare head-to-head comparison (Reference Brown, McElroy and KeckBrown 2006). However, olanzapine alone appears to be less effective than in combination with fluoxetine, although the combination has not been tested extensively (Table 1, Fig. 1). Not surprisingly, both olanzapine and quetiapine (which have antimanic properties) have yielded somewhat lower risks of mood switching than with placebo (Reference Selle, Schalkwijk and VázquezSelle 2014).
The number needed to treat for olanzapine plus fluoxetine and quetiapine alone was <6 (Reference Cookson, Keck and KetterCookson 2007; Reference Selle, Schalkwijk and VázquezSelle 2014). In addition, based on drug–placebo differences in symptomatic improvement, olanzapine plus fluoxetine appeared to be somewhat more effective (22.1% response rate) than quetiapine (16.2%), and substantially more effective than olanzapine alone (7.25%) or lamotrigine (4.80%) (Reference Selle, Schalkwijk and VázquezSelle 2014). Nevertheless, all of these responses are modest.
Only some antipsychotic agents appear to reduce symptoms of acute depression in bipolar disorder, so such responses are evidently not a class effect of all antipsychotics (Table 1, Fig. 1). Supporting this conclusion, the effects of olanzapine alone have been weak although little-studied, aripiprazole was ineffective in a single trial, and other antipsychotic agents remain to be evaluated adequately (Reference Selle, Schalkwijk and VázquezSelle 2014). In addition, in effective doses, antipsychotics may cause adverse effects that may not be well tolerated by some patients, particularly excessive sedation and akathisic restlessness (Reference Tamayo, Zarate and VietaTamayo 2010). Moreover, risks of weight gain, type 2 diabetes and other features of metabolic syndrome (e.g. hyperlipidemia, hypertension) are encountered with the use of some antipsychotic drugs (particularly olanzapine and quetiapine), sometimes within 3 months (Reference McElroy, Weisler and ChangMcElroy 2010; Reference Young, McElroy and BauerYoung 2010; Reference Centorrino, Masters and TalamoCentorrino 2012). These medically important adverse effects would tend to limit the potential (though as yet unproven) value of antipsychotic drugs for prophylactic treatment against recurrences of depression in bipolar disorder (Reference BaldessariniBaldessarini 2013a).
In summary, quetiapine, as well as olanzapine combined with fluoxetine (but not alone), and probably lurasidone (which, unlike most antipsychotic drugs, is not proved to have antimanic efficacy), appear to be efficacious in acute bipolar depression, although with some risks, and without compelling evidence for long-term prophylactic effects for bipolar depression.
Experimental treatments
Other treatments for bipolar depression remain experimental or lack regulatory approval (Reference Poon, Sim and SumPoon 2012; Reference Vázquez, Tondo and UndurragaVázquez 2013). We discuss those with the most developed evidence in this section.
Drugs with mood-elevating effects
Antidepressants, although widely used despite lack of explicit FDA approval for bipolar depression (even though ambiguously approved for major depressive episodes), have inconsistent support from controlled trials, as discussed earlier. In addition, lithium carbonate lacks explicit FDA approval for the treatment of acute depression in bipolar disorder, and it lacked efficacy in one trial not necessarily optimised to test lithium (Table 1, Fig. 1). Other agents with mood-elevating effects, notably stimulants such as methylphenidate and amphetamines and anti-narcolepsy agents such as modafinil and R-modafinil, as well as centrally active dopaminergic agonists such as pramipexole and ropinirole developed for the treatment of Parkinson’s disease, also remain experimental.
Anticonvulsants
In addition, several anticonvulsants lack specific evidence of efficacy in acute bipolar depression (e.g. gabapentin, levetiracetam) or have some research support but lack regulatory approval (e.g. carbamazepine, lamotrigine, pregabalin, valproate) (Table 1, Fig. 1).
Second-generation antipsychotics
Second-generation antipsychotics that appear to be ineffective or lack adequate testing for bipolar depression include asenapine, aripiprazole, clozapine, iloperidone, paliperidone, risperidone and ziprasidone. Older antipsychotics are usually avoided in bipolar disorder owing to their adverse effects.
Other pharmacological treatments
The possible value of other pharmacological treatments has been considered, including calcium channel blockers, anticholinesterases, omega-3 fatty acids and other ‘nutriceuticals’, as well as exogenous thyroid hormones, but they require further testing in bipolar depression (Reference Poon, Sim and SumPoon 2012). Promising findings have been recently reported for the glutamate N-methyl-D-aspartate receptor antagonist ketamine, which is inconvenient to administer (orally inactive) and short-acting. Similar agents, including S-ketamine, memantine and riluzole, remain to be tested and developed into clinically practical treatments with sustained benefits and, ideally, oral activity (Reference Mathews and ZarateMathews 2013).
Non-pharmacological treatments
Non-pharmacological treatments may also be of value, or remain experimental. In particular, psychotherapies continue to be used widely even without adequate evidence of efficacy in bipolar depression, probably by hopeful analogy to the proven value of methods such as cognitive–behavioural and interpersonal psychotherapies in unipolar major depressive disorder (Reference Schaub, Neubauer and BernhardSchaub 2013) and evidence that psychosocial interventions augment mood stabilisation in bipolar disorder (Reference Prasko, Ociskova and KamaradovaPrasko 2013). Intense light therapy and sleep deprivation, too, are plausible candidate treatments but require adequate testing in bipolar depression.
Electroconvulsive therapy is probably effective in bipolar depression (Reference Medda R Mauri and FrattaMedda 2013). Vagal nerve stimulation is FDA-approved for otherwise treatment-resistant depression without specification of illness polarity (Reference BaldessariniBaldessarini 2013a). Repeated transcranial magnetic stimulation and various forms of electrical stimulation of the brain remain experimental (Reference Nierenberg, Alpert and Gardner-SchusterNierenberg 2008).
Conclusions
This overview was stimulated by the fact that depression in bipolar disorder is a clinically and economically burdensome, and sometimes dangerous, condition that presents major therapeutic challenges. Episodes of major depression in bipolar disorder, as well as dysthymia and dysphoric/agitated mixed states, are major contributors to residual morbidity, disability and excess mortality, even with treatment.
We summarised the limited research evidence of efficacy for several treatment modalities, finding encouraging results with some mood-stabilising anticonvulsants and second-generation antipsychotic drugs. However, a critical conclusion is that placebo-controlled trials of all plausible treatments in acute bipolar depression remain very scarce, notably including assessment of the oldest and best-established mood-stabilising agent, lithium. The value and risks of antidepressants in acute bipolar depression remain controversial, and research results are inconsistent. Nevertheless, trials that were identified yielded evidence of significant overall efficacy of antidepressants in bipolar depression that, remarkably, was not less than that found in unipolar depression (Table 1).
Moreover, adequately designed assessments of the value and risks of sustained, long-term treatment in bipolar disorder with antidepressants – either alone or more often in combination with mood-stabilising or antipsychotic treatments, with prophylactic intent – remain rare and insufficient to guide clinical practice. The paucity of compellingly effective alternatives encourages continued study of antidepressants in bipolar depression, and the value and potential added safety of their use in combination with mood-stabilising or antipsychotic agents require adequate testing. It is inevitable that lack of highly effective treatments for bipolar depression encourages widespread empirical applications of polytherapy, even though these remain largely untested for effectiveness and safety.
Further studies are required to advance therapeutic practices, despite difficulties that may be encountered, including concerns for potentially dangerous behavioural activation during antidepressant trials. There is also uncertain commercial interest in bipolar depression, as distinct from large and proven markets represented by unipolar major depressive and anxiety disorders, as well as concern for risk of inducing mania (which is unlikely with antipsychotic agents). Another important but unresolved question is whether particular aspects of psychopathology – including mild, subsyndromal hypomanic features or elements of mixed states that would not meet currently widely used but narrow diagnostic criteria – are predictive of poor responses to antidepressants in depression, as has been proposed recently. Moreover, it remains unclear whether such an effect would represent a characteristic of a syndrome type or a consequence of current agitation.
Also needed is further clarification of the efficacy and safety of antidepressants in varying doses for different forms of depressive morbidity in bipolar type I and II disorder, as well as for the emerging bipolar spectrum of disorders marked by recurrent depression and mild hypomanic features. Specifically, head-to-head comparisons are needed to compare the short- and long-term efficacy and safety of antidepressants and other medicines found to be effective in bipolar depression against each other and against lithium, administered both in monotherapy and in controlled combinations. Important additional concerns include whether some agents proposed for treating bipolar disorder may worsen aspects of mood, functional status or general health (e.g. excessive sedative effects or weight gain, metabolic syndrome).
In summary, we continue to be struck by the disparity between the seriousness of bipolar depression as an unresolved therapeutic challenge and the limited research effort that has been given to its experimental and clinical treatment over the past half-century of research into major mood disorders.
MCQs
Select the single best option for each question stem
-
1 Regarding treatment of bipolar depression with antidepressants:
-
a most expert guidelines recommend them as first-choice monotherapy
-
b they should always be avoided
-
c they are more effective long-term than short-term, especially when added to lithium or an anticonvulsant
-
d in practice, they are the most prevalent treatment provided to patients with bipolar disorder
-
e they are about as well studied in bipolar as in unipolar major depression.
-
-
2 Which of the following has been associated with greatest risk of manic/hypomanic mood switching:
-
a tricyclic antidepressants
-
b selective serotonin reuptake inhibitors
-
c monoamine oxidase inhibitors
-
d venlafaxine
-
e both a and d.
-
-
3 Which has yielded favourable number needed to treat estimates of <6:
-
a olanzapine plus fluoxetine
-
b olanzapine alone
-
c lamotrigine
-
d paroxetine
-
e both a and c.
-
-
4 Which lacks evidence of efficacy in acute bipolar depression:
-
a aripiprazole
-
b paliperidone
-
c gabapentin
-
d only a and c
-
e all of the above.
-
-
5 Which of the following statements is false:
-
a therapeutics research is very limited regarding acute bipolar depression and nearly lacking for long-term, prophylactic benefits and risks
-
b only 4 small trials (<100 patients) support the apparent value of divalproex as monotherapy for acute bipolar depression
-
c there is little information concerning the effects of lithium in acute bipolar depression, despite its use as a fundamental treatment for bipolar disorder for more than six decades
-
d quetiapine 600 mg/day was significantly more effective than 300 mg/day in at least 2 controlled trials
-
e beneficial effects are not a universal or class effect of all modern antipsychotic agents.
-
MCQ answers
| 1 | d | 2 | e | 3 | a | 4 | e | 5 | d |





eLetters
No eLetters have been published for this article.