Introduction
As a significant emblem of premium livestock offerings, the consumer interest in pigeon squab is experiencing a surge (Ji. et al. Reference Ji, Zhang and Shao2020). However, in their natural state, the reliance of pigeon squabs on their parents for nourishment post-hatching poses a considerable limitation on the efficiency of production (Jin et al. Reference Jin, Y. and Jiang2023; Mahdy Reference Mahdy2021). Early weaning (EW) and artificial rearing appear to be the optimal strategies for mitigating the pressure of parental feeding and overcoming this production challenge (Xu. et al. Reference Xu, M. and Yuan2024). Similar to mammals, EW means that young pigeons leave their dependent living environment, which can cause severe stress both psychologically and physiologically. The stress of EW in mammals has been extensively studied. Smith (Smith and Clark Reference Smith and Clark2010) and Hu (Hu et al. Reference Hu, Xiao and Luan2013), among others, have discovered that weaning stress results in damage of the intestinal mucosal barrier, diarrhea, and dysbiosis in piglets, which are also attributed to the direct inhibition of growth in these young animals. Research on weaning stress in young pigeons is quite limited. Preliminary studies by our group indicate that the damage to the intestinal epithelial barrier function accompanying EW stress might be a primary factor contributing to the slow growth rate observed in pigeon squabs post-weaning (Xu et al. Reference Xu, Jian and Zhao2022).
Naive CD4+ T-cells can be differentiated into T helper cell (Th) subsets (Th1/Th2/Th17) or regulatory T cell (Treg) on the basis of cytokines in their milieu (Pagani et al. Reference Pagani, Rossetti and Panzeri2013). It is well characterized that balances of Th1/Th2 and/or Th17/Treg are critical for the homeostatic maintenance of immune responses (Gyun et al. Reference Gyun, Zi and Min2020). Moreover, studies in humans and mice have shown that the integrity of intestinal epithelial barrier is essential for homeostatic regulation (Cohen and Denkers Reference Cohen and Denkers2014; Kayama and Takeda Reference Kayama and Takeda2012). For instance, macromolecular antigens in the intestinal cavity can be leaked out and captured by T lymphocytes when the integrity of intestinal epithelial barrier is broken, leading to Th subsets imbalance (Chen Reference Chen2021). Therefore, a novel hypothesis has been proposed that the intestinal epithelial barrier damage induced by EW might be associated with an imbalance in Th cell differentiation in squabs. However, there are currently no data available on the Th subsets imbalance in pigeon squabs with damaged intestinal epithelial barrier function.
The purpose of this study was to elucidate the intrinsic connection between the damage to the intestinal epithelial barrier function induced by EW and the immune imbalance of Th1/Th2 and Th17/Treg by analyzing the changes in growth performance, intestinal permeability, gene expression of tight junction proteins, ileal cytokine concentrations, and mRNA expression of ileal Th1, Th2, Th17, and Treg-specific transcription factors during the development process of squabs undergoing EW. Understanding the changes in gut barrier function and immunity due to weaning stress could provide potential possibilities for mitigating the damage induced by weaning stress in squabs.
Materials and methods
All animal-related experimental procedures were greenlit by the Animal Care and Welfare Committee of Animal Science College and the Scientific Ethical Committee of Zhejiang University (Hangzhou, China).
Animals and experiment layout
On the day of emergence, 160 newly born White King pigeon squabs (Columba livia) with comparable body masses were chosen. They were matched up and distributed to the nests of the parent pigeons, replacing the artificial eggs that were previously used to accommodate the nesting instincts of the parent birds. Parent pigeons were fed a grain-based diet. The dietary composition is shown in Supplementary Table 1. Each pair of parent pigeons nurtured two squabs, which were nourished with pigeon milk directly from their parents’ beaks to their own. Seven days post-hatching, the squabs with similar body weight (89.39 ± 1.91 g) were randomly allocated into two groups: a control (CON) group and an EW group. Each group consisted of 8 replicates, with 10 squabs in each replicate. The squabs in the CON group continued receiving nourishment from their parent pigeons, whereas the squabs in the EW group were separated from their parents and provided with artificial pigeon milk. The formula of the artificial pigeon milk, which includes 17.76% protein and 13.07 MJ/kg of energy, is shown in Supplementary Table 2. The artificial pigeon milk powder was blended with lukewarm water at a temperature of 37°C and administered into the crop via a sterile syringe and tube for simulated feeding. The environmental settings for both groups were kept consistent. Throughout the duration of the study, the surrounding temperature was maintained between 18 and 26°C, the relative humidity was held steady at 60–70%, and the lighting cycle was set to 12 h of light followed by 12 h of darkness.
Sample gathering
On days 1 (D1), 4 (D4), 7 (D7), 10 (D10), 14 (D14) and 21 (D21) following weaning, eight squabs (one from each replicate) were randomly chosen from both the CON and EW groups for collection. All the squabs were measured for weight and then humanely euthanized through cervical displacement (those exceeding 250 g were sedated prior to cervical displacement). The squabs were then sacrificed to obtain serum and small intestine samples. Blood samples were taken for the analyses of serum endotoxin, diamine oxidase, and d-lactic acid levels. The ileum mucosa was gathered to determine the gene expression of Th1, Th2, Th17, and Treg cell-specific transcription factors and tight junction proteins, along with the cytokine levels.
Intestinal permeability
The levels of endotoxin, diamine oxidase, and d-lactate in serum were assessed using a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA), in conjunction with commercial ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China), adhering to the protocol provided by the manufacturer.
Cytokine concentration assessment
Ileal mucosa homogenates were prepared using phosphate-buffered saline for the assessment of cytokine concentrations. The concentrations of interleukin-1β (IL-1β), IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-17, IL-23, tumor necrosis factor-α (TNF-α), transforming growth factor (TGF-β), and interferon-γ (IFN-γ) were quantified using commercial ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). Standard samples and diluent were dispensed at a volume of 100 μL per well in duplicate. The plate was then incubated for 2 h at 37°C and subsequently washed thrice with a washing solution. A biotin-labeled antibody was introduced at a volume of 100 μL per well, followed by incubation at 37°C for 1 h and washed five times. A horseradish peroxidase conjugate was then applied at 100 μL per well, incubated at 37°C for 1 h, and washed five times. Following this, 100 μL of the coloring reagent was added to each well to develop the color for 15 min at 37°C. To terminate the reaction, 50 μL of stop solution was introduced into each well. The changes in absorbance at a wavelength of 450 nm were promptly measured using a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The cytokine concentrations were ultimately expressed as picograms per milligram of protein.
RNA extraction and real-time quantitative PCR analysis
Total RNA was extracted from ileal mucosa utilizing the MolPure® TRleasy™ Plus Total RNA Kit (with spin column) (Yeasen Biotechnology Co., Ltd., Shanghai, China) following the protocol of the manufacturer. The quality of the total RNA was evaluated through 1% denaturing agarose gel electrophoresis, while its concentration and purity were determined with the NanoDrop 2000 (Thermo Fisher, Waltham, MA, USA). The OD260/OD280 ratio ranged from 1.8 to 2.0, indicating that the RNA isolate was of high purity and suitable for further tests. Reverse transcription to obtain cDNA was performed utilizing the Hifair® III 1st Strand cDNA Synthesis SuperMix for qPCR (gDNA digester plus) (Yeasen Biotechnology Co., Ltd., Shanghai, China). The abundance of mRNA was detected using the Bio-Rad CFX Manager 3.0 system (CFX96 Touch, Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primers for both the reference and target genes are detailed in Table 1. Real-time quantitative PCR was performed using the Hieff® qPCR SYBR Green Master Mix (No Rox; Yeasen Biotech Co., Ltd., Shanghai, China), with the thermal cycling program set as follows: initial DNA denaturation at 95°C for 5 min, succeeded by 40 cycles of denaturation at 95°C for 10 s and annealing/extension at 60°C for 30 s. The relative expression levels of each gene were computed employing the 2-ΔΔCt approach (Schmittgen Reference Schmittgen2001), with GAPDH as endogenous reference and the mean ΔCt value of the CON group as calibrator.
Table 1. Targeted gene primers employed in quantitative real-time PCR analysis
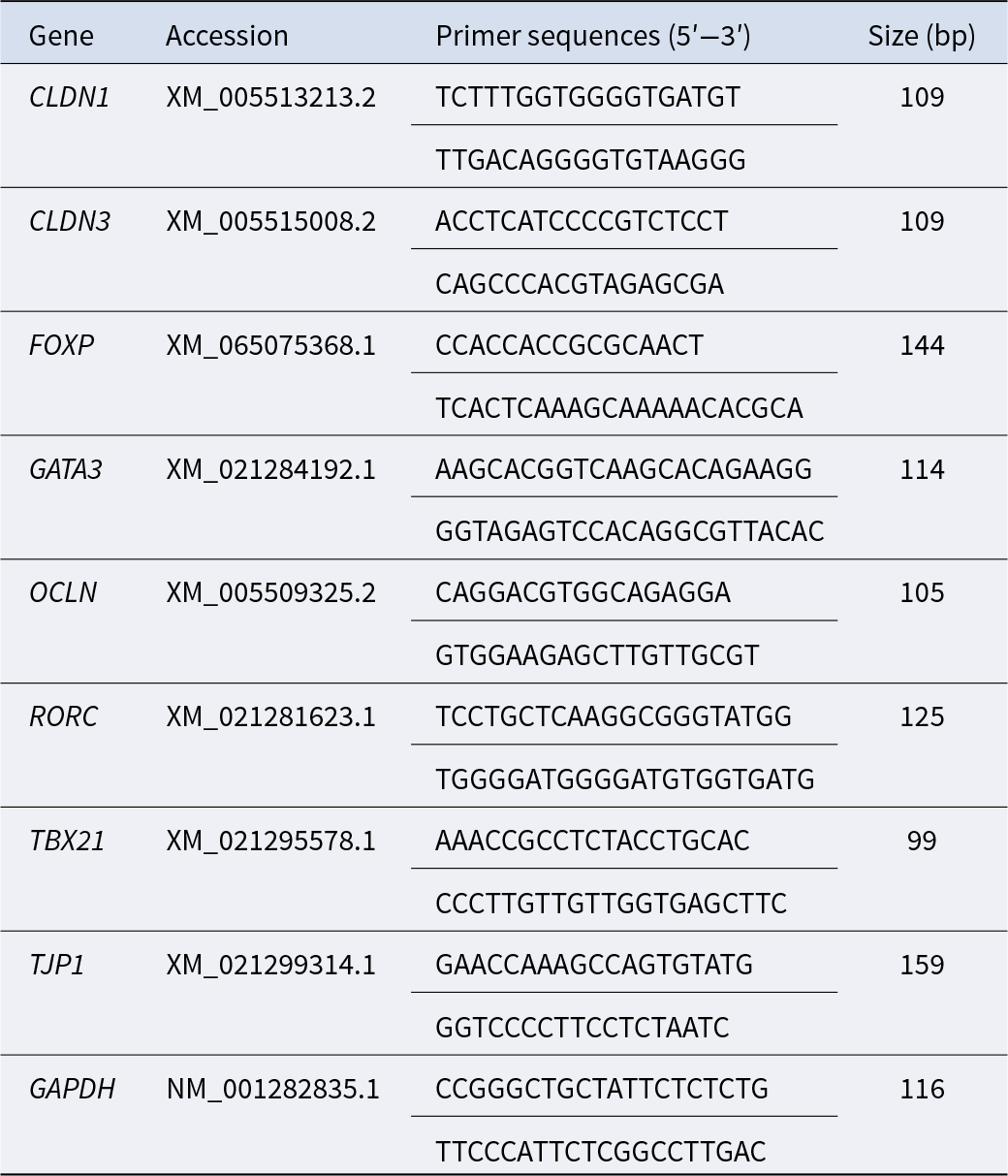
Note: CLDN, claudin; FOXP, forkhead box protein P; GATA3, GATA binding protein 3; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; OCLN, occludin; RORC, RAR-related orphan receptor C; TBX21, T-box 21; TJP1, tight junction protein 1.
Statistical examination
The experimental data were analyzed using an independent-samples t-test in SPSS 26.0 (SPSS Inc., Chicago, IL, USA) for Windows. Statistical significance was set at P < 0.05, while a higher threshold for significance was designated at P < 0.01. Data visualization was achieved using GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA). The results are expressed as means accompanied by their standard errors for a group of eight squabs. Mantel tests were carried out utilizing the R software suite (Hy4m/linkET). The asterisks were used to denote statistical significance as follows: an asterisk (*) denotes a significant difference (P < 0.05), while double asterisks (**) signify a highly significant difference (P < 0.01).
Results
Body weight
The effects of EW on body weight of squabs are depicted in Figure 1. The body weight of early weaned squabs at different time points (D1, D4, D7, D10, D14, and D21 post-weaning) were markedly decreased (P < 0.01) in comparison to that of CON group.
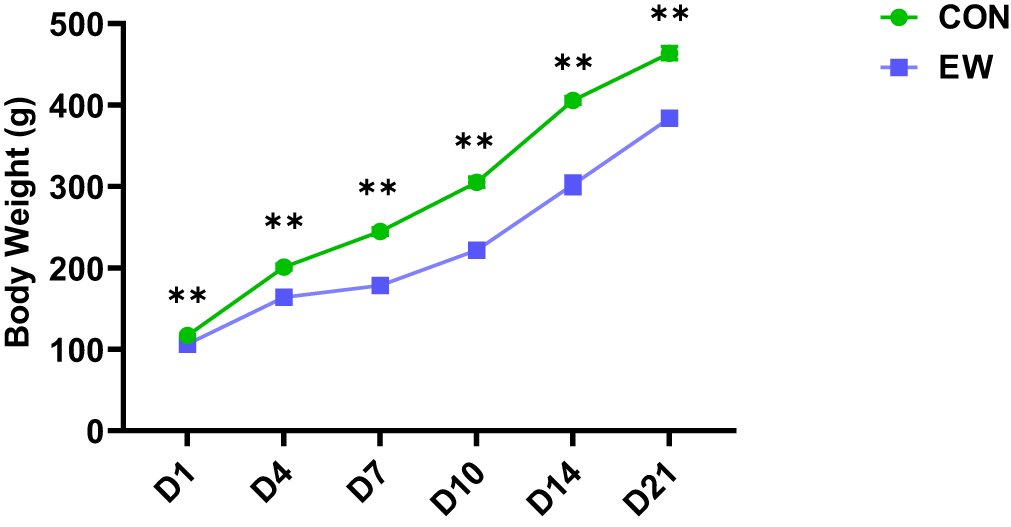
Figure 1. Effects of early weaning on body weight of squabs. The 1D, 4D, 7D, 10D, 14D, and 21D represent the 1st, 4th, 7th, 10th, 14th, and 21st day after weaning, respectively. Values are means with their standard errors of eight squabs. **p < 0.01, respectively, within each time points post-weaning. CON, control group; EW, early weaning group.
Intestinal permeability
The consequences of EW on the intestinal permeability in squabs are illustrated in Figure 2. The intestinal permeability in squabs was indicated by serum levels of diamine oxidase, endotoxin, and d-lactic acid. EW led to a notable elevation in serum levels of endotoxin and diamine oxidase in squabs at D4, D7, D10, D14, and D21 following weaning (P < 0.01, P < 0.01, P < 0.05, P < 0.05, and P < 0.05, respectively). Additionally, the serum d-lactic acid level was significantly elevated (P < 0.05) in early weaned squabs at D4 post-weaning relative to the CON group, whereas no significant difference (P > 0.05) in serum d-lactic acid level was observed between the two groups at the other time points.
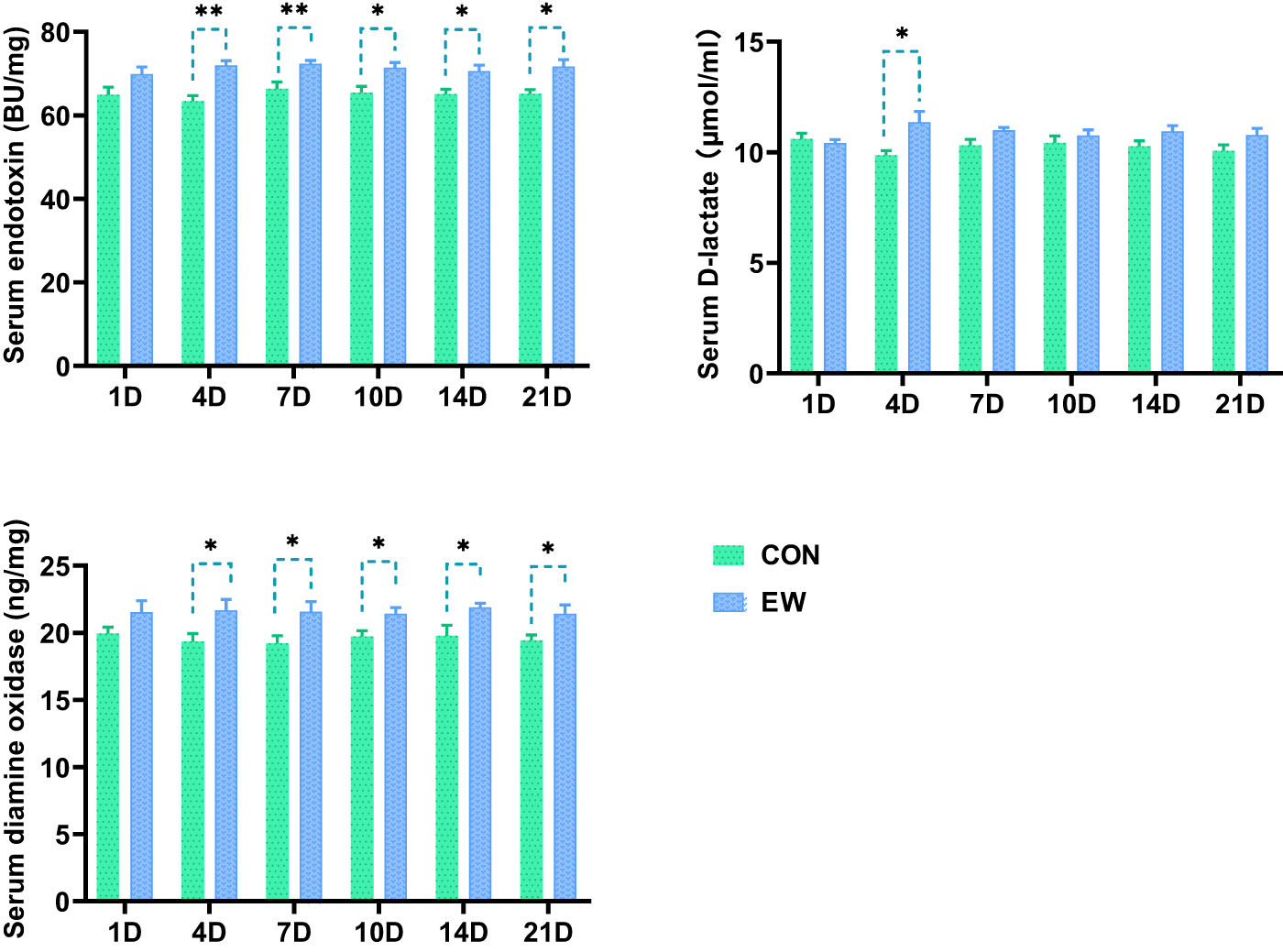
Figure 2. Effects of early weaning on the levels of serum endotoxin, d-lactate and diamine oxidase of squabs. The 1D, 4D, 7D, 10D, 14D, and 21D represent the 1st, 4th, 7th, 10th, 14th, and 21st day after weaning, respectively. Values are means with their standard errors of 8 squabs. *p<0.05 and **p < 0.01, respectively, within each time points post-weaning. CON, control group; EW, early weaning group.
Relative gene expression of tight junction proteins
The impacts of EW on the relative gene expression of tight junction proteins in ileum of squabs are presented in Fig. 3. In comparison to the CON group, the mRNA expression of OCLN and CLDN1 in the EW group was significantly downregulated from 4 to 21 days after weaning (P < 0.05 or P < 0.01). The mRNA expression of TJP1 was markedly downregulated in early weaned squabs at D10 and D14 after weaning (P < 0.05, P < 0.01). The mRNA expression of CLDN3 was markedly downregulated from D1 to D21 post-weaning (P < 0.05 or P < 0.01).
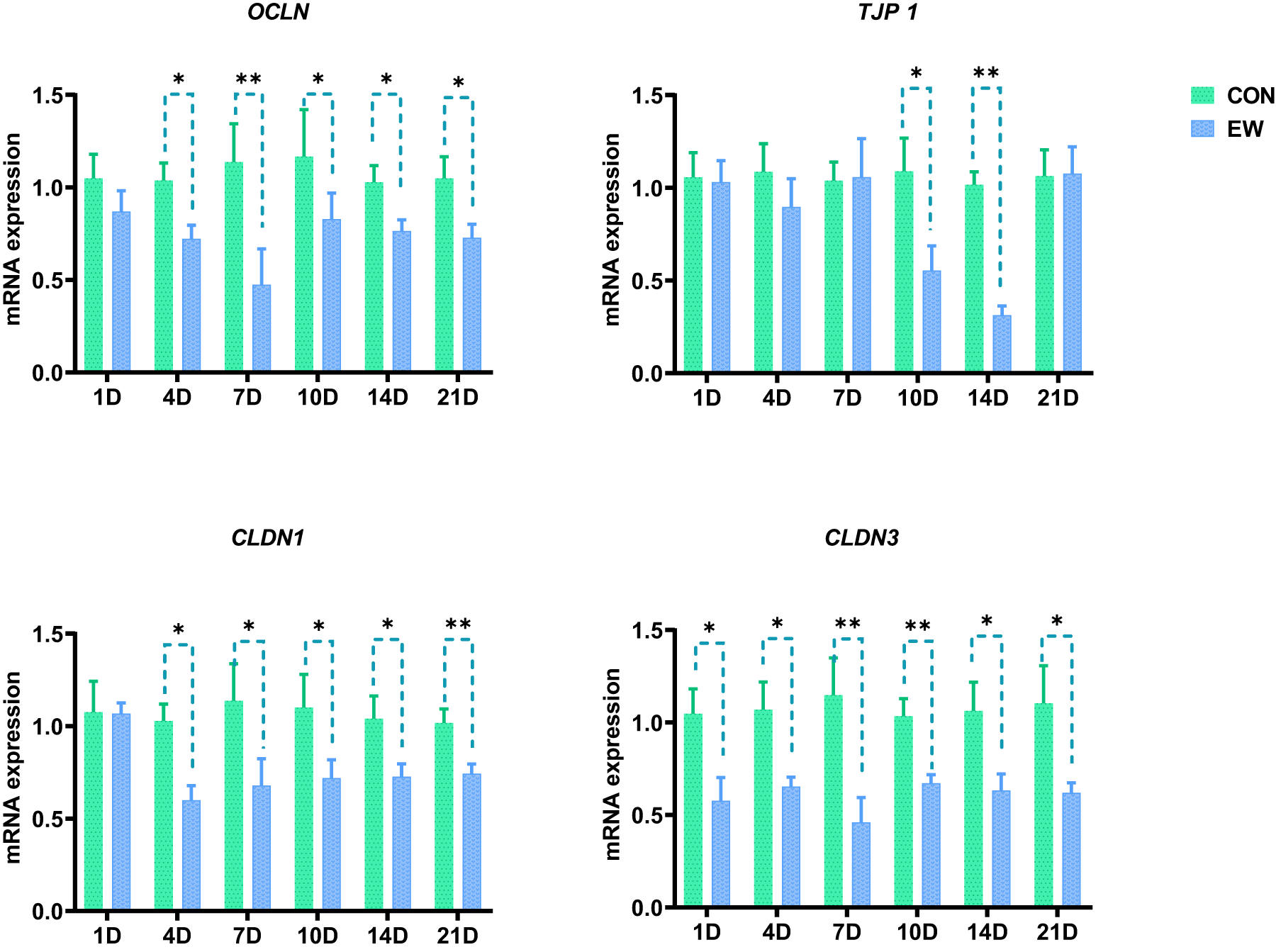
Figure 3. Effects of early weaning on the mRNA expression of tight junction proteins in ileum of squabs. CLDN, claudin; OCLN, occludin; TJP, tight junction protein. The 1D, 4D, 7D, 10D, 14D, and 21D represent the 1st, 4th, 7th, 10th, 14th, and 21st day after weaning, respectively. *p < 0.05 and **p < 0.01, respectively, within each time points post-weaning. CON, control group; EW, early weaning group.
Concentrations of Th cell subset differentiation-inducing factors
Inexperienced CD4+ T cells, referred to as Th0 cells, have the capacity to mature and proliferate into diverse subsets including Th1, Th2, Th17, and Treg under the influence of distinct cytokines. Upon exposure to IL-12 and IL-4, Th0 cells can differentiate into Th1 and Th2 cells, respectively. In the presence of TNF-α, IL-6, IL-1β, and IL-23, they tend to develop into Th17 cells. When acted upon by TGF-β alone, Th0 cells differentiate into Treg cells. However, when exposed to both TGF-β and IL-6, Th0 cells are steered toward differentiation into Th17 cells instead.
The concentrations of Th1, Th2, Th17, and Treg differentiation-inducing factors in ileum of the squabs in the CON and EW groups are displayed in Figure 4. In comparison to the CON group, the EW group presented a significant rise in IL-12 level at D10 and D21 after weaning (P < 0.05). The IL-4 level markedly reduced in the early weaned squabs from 14 to 21 days post-weaning (P < 0.05), while the levels of IL-1β and IL-23 significantly increased in the early weaned squabs from 4 to 21 days post-weaning (P < 0.05). Additionally, the levels of IL-6 and TNF-α significantly increased in the early weaned squabs from 7 to 21 days post-weaning (P < 0.05 or P < 0.01). Besides, the TGF-β level significantly decreased in the EW group from 1 to 21 days post-weaning (P < 0.05).

Figure 4. Effects of early weaning on the levels of differentiation-inducing factors (IL-1β, IL-4, IL-6, IL-12, IL-23, TNF-α, TGF-β) of Th1, Th2, Th17, and Treg in ileum of squabs. The 1D, 4D, 7D, 10D, 14D, and 21D represent the 1st, 4th, 7th, 10th, 14th, and 21st day after weaning, respectively. *p < 0.05 and **p < 0.01, respectively, within each time points post-weaning. CON, control group; EW, early weaning group.
Gene expression of Th cell subset-specific transcription factors
T-bet, GATA3, RORγt, and FOXP serve as specific transcription factors which control the differentiation of Th0 cells into Th1, Th2, Th17, and Treg subtypes, respectively. The genes TBX21, GATA3, RORC, and FOXP are responsible for encoding these four transcription factors. The changes in the mRNA expression of the transcription factors for Th1, Th2, Th17, and Treg cells within 21 days post-weaning in pigeon squabs are presented in Figure 5. In the EW group, the mRNA expression of the transcription factor RORC was significantly elevated compared to the CON group from D4 to D14 post-weaning (P < 0.05 or P < 0.01), while the mRNA expression of GATA3 and FOXP was significantly reduced compared to the CON group at D4 and from D10 to D21 post-weaning (P < 0.05). There was no notable variation in the TBX21 mRNA expression between these two groups (P > 0.05).

Figure 5. Effects of early weaning on the mRNA expression of specific transcription factors of Th1, Th2, Th17, and Treg in ileum of squabs. The 1D, 4D, 7D, 10D, 14D, and 21D represent the 1st, 4th, 7th, 10th, 14th, and 21st day after weaning, respectively. *p < 0.05 and **p < 0.01, respectively, within each time points post-weaning. TBX21, T-box transcription factor 21; GATA3, GATA binding protein 3; RORC, RAR-related orphan receptor C; Foxp, forkhead box protein P; CON, control group; EW, early weaning group.
Concentrations of effector cytokines of Th cell subsets
Th1 cells primarily secrete cytokines such as INF-γ and TNF-α; Th2 cells chiefly produce cytokines like IL-5, IL-6, and IL-13; Th17 cells predominantly secrete cytokines including IL-17 and IL-22; while Treg cells mainly release cytokines such as IL-10 and TGF-β. The cytokines secreted by Th1, Th2, Th17, and Treg in ileum of squabs in the CON and EW groups were mainly depicted in Figure 6. In comparison to the CON group, the levels of IFN-γ and TNF-α (Figure 4) in the EW group were markedly increased from 7 days after weaning (P < 0.05). In comparison to the CON group, the IL-6 (Fig. 4) level in the EW group was markedly increased from 7 days after weaning (P < 0.05), while the IL-5 level was markedly reduced in the EW group from 7 days after weaning (P < 0.05). Besides, the IL-13 level had no significant change at each time point after weaning (P > 0.05). The IL-17 in the EW group was markedly increased from 7 days after weaning (P < 0.05), and the IL-22 level was markedly increased in the EW group from 10 days after weaning (P < 0.05). Besides, the IL-10 level was markedly reduced in the EW group from 10 days after weaning (P < 0.05). The TGF-β (Figure 4) level was markedly decreased in the EW group from 1 day after weaning (P < 0.05).
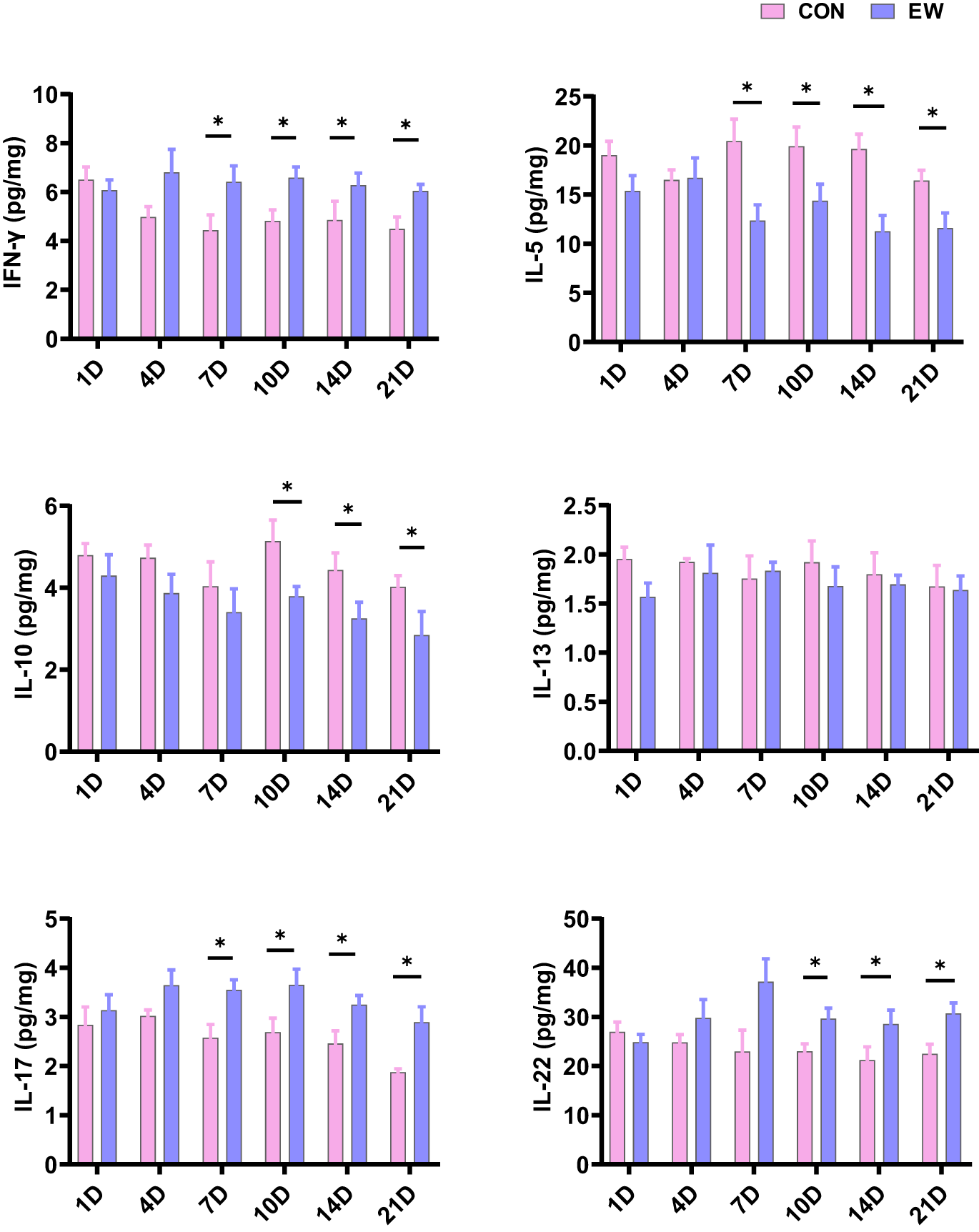
Figure 6. Effects of early weaning on the secretion of cytokines (IFN-γ, IL-5, IL-10, IL-13, IL-17, IL-22) by Th1, Th2, Th17, and Treg cells in ileum of squabs. The 1D, 4D, 7D, 10D, 14D, and 21D represent the 1st, 4th, 7th, 10th, 14th, and 21st day after weaning, respectively. *p < 0.05 within each time points post-weaning. CON, control group; EW, early weaning group.
Correlation analysis
Potential associations between factors related to Th cell subset differentiation balance and indicators related to intestinal epithelial barrier function were evaluated based on Mantel tests (Figure 7). The results showed that during EW, the changes of differentiation-related factors of Th17/Treg cell subsets were significantly correlated with the levels of serum diamine oxidase, serum endotoxin, and the mRNA expression of CDLN1 in ileum (P< 0.01). The Th1/Th2 cell subsets differentiation-related factors were significantly correlated with serum endotoxin level (P< 0.05).
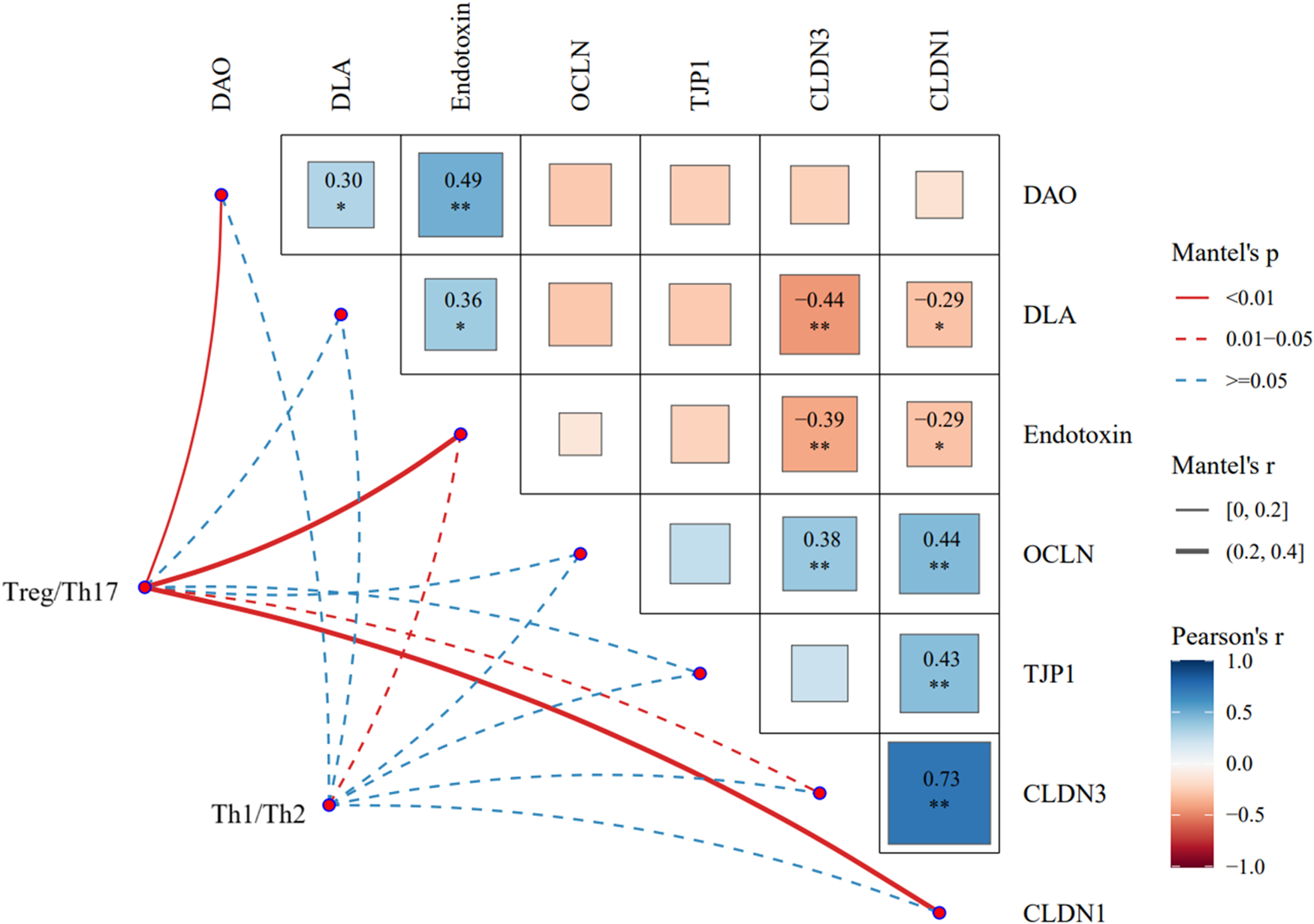
Figure 7. Correlation of Th1, Th2, Th17, and Treg with epithelial barrier function was evaluated based on mantel test. CLDN, claudin; OCLN, occlude; TJP, tight junction protein; DAO, diamine oxidase; DLA, d-lactate.
Discussion
In studies on mammals, it has been found that the sudden dietary switch from breastfeeding to solid nourishment after weaning could cause insufficient intake in young animals, which may subsequently result in inadequate utilization of feed nutrients, localized inflammatory reactions and oxidative stress, excessive proliferation of harmful bacteria, diarrhea, suboptimal growth, and potentially mortality (Campbell et al. Reference Campbell, Crenshaw and Polo2013; Yang et al. Reference Yang, Wang and T.2021). The phenomenon of growth inhibition also occurred in the weaned squabs in our study. The outcomes revealed that the growth performance of the early weaned squabs conspicuously lagged behind that of the parent-fed squabs as early as 1 day post-weaning. The nutrient absorption primarily occurs in the intestine, and a well-functioning intestine plays a pivotal role in promoting animal growth and development while serving as a critical physiological foundation for enhancing immune competence (Greenwood-Van et al. Reference Greenwood-Van, Johnson and Grundy2017; Pluske et al. Reference Pluske, Turpin and Kim2018). Similar to mammals, squabs also experienced a sudden change in environment and diet during weaning. Therefore, the damage to the intestinal structure and immune function caused by weaning stress might be a direct reason for the growth impairment observed in weaned pigeons. An important target in current nutritional studies is to ensure the maintenance of the intestinal structural integrity and its standard functionality (Cui et al. Reference Cui, Guan and Ding2021; Guo et al. Reference Guo, Geng and Chen2021). Therefore, the study further investigated the implications of EW on the intestinal epithelial barrier and immune function in squabs. Serum diamine oxidase, d-lactic acid, and endotoxin levels are commonly used to reflect intestinal permeability (Xu. et al. Reference Xu, M. and Yuan2024). The findings of the current research showed that at one or multiple age in days post-weaning, there was a significant boost in the levels of serum diamine oxidase, d-lactic acid, and endotoxin, pointing to a rise in intestinal permeability in weaned squabs. Notably, the elevated serum endotoxin and diamine oxidase persisted up to 21 days post-weaning, which was in agreement with the findings in the previous study (Xu et al. Reference Xu, Jian and Zhao2022). A sustained elevation in intestinal permeability indicated that the epithelial barrier was compromised in weaned pigeons. Additionally, tight junctions which form a highly polarized barrier are indispensable elements of the intestinal epithelial barrier and are crucial for upholding the internal environment stable. Impaired synthesis or reduced activity of these proteins may increase the selective permeability of the intestinal epithelial barrier (Chen et al. Reference Chen, Naehrer and Applegate2016). The outcomes of the present investigation demonstrated a significant downturn in the mRNA expression of OCLN, CLDN1, CLDN3, and TJP1 in the EW group, with low mRNA expression of OCLN, CLDN1, and CLDN3 being persistent. These findings further illustrated that the intestinal epithelial barrier in weaned pigeons suffered damage to its integrity, and the injury was long-term, not improving with age.
The development of the intestinal mucosal immune system represents the maturation process of both innate and acquired immunity. The physical defense provided by the intestinal epithelial lining, the protective mucosal coat, complement, antimicrobial peptides, and the cytokines released by resident immune cells like macrophages and dendritic cells collectively constitute the innate immune system (Malago Reference Malago2015). CD4+ T cells serve as pivotal intermediaries and crucial regulators of adaptive immunity, with their differentiation pathways being controlled by various factors including antigen-specific stimulation, cytokines, and transcription factors (Gyun et al. Reference Gyun, Zi and Min2020). Depending on the cytokine expression profiles and differences in immunoregulatory functions, effector T cells derived from CD4+ T cell differentiation are categorized into distinct functional subsets (Shale et al. Reference Shale, Schiering and Powrie2013). These subsets include Th1, Th2, Th17, and Treg, among others, which coordinate with each other to collectively exert immunoregulatory effects. It is commonly believed that Th1 and Th17 subsets, along with their associated cytokines, play pro-inflammatory roles, while Th2 and Treg subsets, along with their respective cytokines, exert anti-inflammatory effects. The dynamic balance between Th1/Th2 and Th17/Treg subsets is considered a crucial mechanism for maintaining immune stability (Aiping et al. Reference Aiping, Nonghua and Hao2010; Fang et al. Reference Fang, Juan and Chao2019). Once this dynamic balance is disrupted, it can lead to or exacerbate intestinal inflammation. On account of the presence of intestinal epithelial barrier, the vast majority of microbes normally do not interact directly with immune cells. Once the intestinal epithelial barrier is damaged, macromolecular antigens such as microorganisms in the intestinal cavity are more likely to leak out, and thus be captured by intraepithelial lymphocytes, leading to the imbalance of Th cell differentiation (Chen Reference Chen2021). Thus, we hypothesize that the intestinal epithelial barrier damage induced by EW might be associated with an imbalance in Th cell differentiation in ileum of squabs. Consequently, the differentiation of four Th cell subsets and the changes of related cytokine networks were measured. The results revealed that in the ileum of weaned squabs, cytokines that induce Th1 and Th17 cell differentiation significantly increased at multiple time points, while cytokines that induce Th2 and Treg differentiation significantly decreased at various points post-weaning. The mRNA expression of GATA3 and FOXP, nuclear transcription factors associated with Th2 and Treg cells, respectively, significantly decreased. Conversely, the mRNA expression of RORC, a nuclear transcription factor associated with Th17 cells, significantly increased. The effector cytokine levels associated with Th1 and Th17 significantly rose, while those associated with Th2 and Treg either dropped or displayed no significant change. Collectively, these findings indicated that post-weaning squabs exhibited a disruption of Th1/Th2 and Th17/Treg immune homeostasis in the ileum. It is noteworthy that the significant changes in the expression of transcription factors were commonly observed at 4 days post-weaning, whereas the significant changes in effector cytokine levels generally became apparent starting at 7 days post-weaning.
Prior research has shown that intestinal inflammation in mice can be mediated by Th1/Th2 cells and is associated with abnormal concentration of IL-1β, IL-6, IL-12, TNF-α, and IFN-γ (Peng Reference Peng2012). Specifically, Th17 cells can mediate Th17-type immunity at the intestinal mucosa by secreting the distinct cytokines IL-22 and IL-17, which in turn induces the occurrence of epithelial inflammation in the intestine (Proietti et al. Reference Proietti, Cornacchione and Rezzonico2014; Ray et al. Reference Ray, De Salvo and Pizarro2014). Treg cells and the specific cytokine IL-10 secreted by Treg cells negatively regulated the mucosal immune process, and at the same time promoted the renewal and remodeling of small intestinal epithelial cells (Mayne and Williams Reference Mayne and Williams2013; Rau et al. Reference Rau, Rehman and Dittrich2018). In short, The cells in the intestine participate in the development of intestinal epithelium and are instrumental in preserving the homeostasis of the intestinal epithelial barrier together with their related cytokines. Presumably, EW is linked to the occurrence of Th1/Th2 and Th17/Treg immune imbalances in small intestine of squabs, which in turn altered the cytokine environment in the intestine, ultimately leading to aggravation of intestinal barrier injury in squabs. To further verify intrinsic connection between the compromise of intestinal epithelial barrier function resulting from EW and the Th1/Th2, Th17/Treg immune imbalance in squabs, Mantel test was employed to assess the correlation between these related factors. The results revealed that during the process of EW, the Th17/Treg imbalance was highly significantly correlated with increased intestinal mucosal permeability and abnormal expression of tight junction proteins. Meanwhile, the Th1/Th2 imbalance was only moderately correlated with a decrease in serum endotoxin level. These suggested that the Th17/Treg imbalance appeared to be more closely associated with the intestinal epithelial barrier impairment in pigeon squabs undergoing EW.
However, two limitations of this study must be taken into consideration. First, it is important to point out that differentiation of naive CD4+ T cells into Th (Th1/Th2/Th17) or Treg cells is influenced by many exogenous and endogenous factors. For example, intestinal commensal bacterial metabolites or bacteria themselves can activate naive CD4+ T cells to effector T cells, Tregs, or Th17 cells by altering cytokines environment through pattern-recognition receptors pathways (Gopalakrishnan et al. Reference Gopalakrishnan, Helmink and Spencer2018). Because several previous studies have shown that the composition of commensal bacterial and their metabolites is influenced by diet composition (Liu et al. Reference Liu, Bian and Sun2017), the effect of diets on Th cell differentiation should not be ignored. As such, it will be necessary to further investigate whether and how diets and microbiota are involved in regulating Th subsets imbalance in pigeon squabs during EW period. Second, we do not elucidate the mechanisms behind the interplay between the Th17/Treg imbalance and the intestinal epithelial barrier function impairment in early weaned squabs. Further studies are required to determine the signal transduction pathways involved. Nevertheless, our results are probably useful for researchers as a first step to understand the potent role of Th subsets in the development and progression of intestinal epithelial barrier function in pigeon squabs under stress.
Conclusion
In conclusion, EW stress could bring about growth retardation in squabs. The intestinal epithelial barrier function was compromised in weaned squabs, primarily manifesting as increased intestinal mucosal permeability, loose tight junctions, and an imbalance in the Th subsets and their cytokine network. Moreover, correlation analysis suggested that the Th17/Treg imbalance might be more closely associated with the damage of intestinal epithelial barrier function in young pigeons undergoing EW.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/anr.2025.6.
Acknowledgements
The study was funded by Zhejiang Provincial Natural Science Foundation of China (grant number ZCLQ24C1701), the Fundamental Research Funds for the Provincial Universities of Zhejiang (grant number 2023YW61), and the Open project of Key Laboratory of Animal Molecular Nutrition of Ministry of Education (Zhejiang University) (grant number KLMAN202301).
Conflict of interest
The contributors state that they have no conflicting interests.










