No CrossRef data available.
Published online by Cambridge University Press: 22 November 2021
Trachoma is an infectious disease and it is the leading cause of preventable blindness worldwide. To achieve its elimination, the World Health Organization set a goal of reducing the prevalence in endemic areas to less than 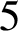 $5$% by 2020, utilizing the SAFE (surgery, antibiotics, facial cleanliness, environmental improvement) strategy. However, in Burundi, trachoma prevalences of greater than
$5$% by 2020, utilizing the SAFE (surgery, antibiotics, facial cleanliness, environmental improvement) strategy. However, in Burundi, trachoma prevalences of greater than 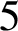 $5$% are still reported in 11 districts and it is hypothesized that this is due to the poor implementation of the environmental improvement factor of the SAFE strategy. In this paper, a model based on an ordinary differential equation, which includes an environmental transmission component, is developed and analysed. The model is calibrated to recent field data and is used to estimate the reductions in trachoma that would have occurred if adequate environmental improvements were implemented in Burundi. Given the assumptions in the model, it is clear that environmental improvement should be considered as a key component of the SAFE strategy and, hence, it is crucial for eliminating trachoma in Burundi.
$5$% are still reported in 11 districts and it is hypothesized that this is due to the poor implementation of the environmental improvement factor of the SAFE strategy. In this paper, a model based on an ordinary differential equation, which includes an environmental transmission component, is developed and analysed. The model is calibrated to recent field data and is used to estimate the reductions in trachoma that would have occurred if adequate environmental improvements were implemented in Burundi. Given the assumptions in the model, it is clear that environmental improvement should be considered as a key component of the SAFE strategy and, hence, it is crucial for eliminating trachoma in Burundi.