Introduction
Loss, deterioration and fragmentation of continuous forest blocks into small, isolated remnants are widely considered as important causes of the current extinction wave in tropical ecosystems (Pimm and Raven Reference Pimm and Raven2000, Brooks et al. Reference Brooks, Mittermeier, Mittermeier, Da Fonseca, Rylands, Konstant, Flick, Pilgrim, Oldfield, Magin and Hilton-Taylor2002, Laurence et al. Reference Laurance, Lovejoy, Vasconcelos, Bruna, Didham, Stouffer, Gascon, Bierregaard, Laurance and Sampaio2002). Through cascade effects, the loss or decline of single species may adversely affect the viability of a suite of other species, both within and between trophic levels (Laurance Reference Laurance, Burslem, Pinard and Hartley2005). For example, local extinction of a single top predator may release the predation pressure on secondary predators, which can then affect the population dynamics of a suite of smaller species (Crooks and Soulé Reference Crooks and Soule1999). At the same time, ecosystems may also be threatened by the introduction or sudden increase in certain species. The introduction of small mammals (e.g. rats, cats) onto oceanic islands by human settlers, for instance, quickly resulted in the (near) extinction of many ground-breeding bird species (Imber et al. Reference Imber, Harrison and Harrison2000, Jones et al. Reference Jones, Williamhenry, Howald, Tershy and Croll2005), which may have offered crucial ecological functions such as seed dispersal and nutrient deposition (Sekercioglu Reference Sekercioglu2006).
When fragmentation progressively increases the edge-to-interior ratio of forest remnants, bird nests are typically exposed to higher predation pressures. Shortly after the destruction of suitable habitat, mobile organisms will take refuge in remaining patches (Goss-Custard et al. Reference Goss-Custard, Caldow, Clarke, Durell, Urfi and West1995, Hagan et al. Reference Hagan, Vander Haegen and McKinley1996). In these terrestrial islands, densities of both breeding birds and their main predators will temporarily increase, which may result in elevated levels of nest predation. But even after bird and predator density have reached an ecological equilibrium, predation rates may remain high in small patches or at forest-field ecotones compared to forest interior. First, the structural diversity of edges provides singing posts, suitable nesting cover and feeding sites for a wide range of bird species, and often results in high nest densities, which may attract nest predators (Gates and Gysel Reference Gates and Gysel1978, Weldon and Haddad Reference Weldon and Haddad2005). Second, both forest and fields may act as a barrier for open field and forest predators respectively, hence, forest-field ecotones may act as ’travel-lanes’ with elevated predator activities (Bider Reference Bider1968, Ferguson Reference Ferguson2000, but see Larivière Reference Larivière2003). Third, species that are associated with habitat edges or the surrounding landscape matrix may invade forest boundaries from the surrounding landscape. This may not only result in increased predation near forest edges, but may additionally increase the competition between forest specialist birds with matrix-dwelling species invading the forest (Janzen Reference Janzen1983, Ness and Morin Reference Ness and Morin2008).
Despite the common belief that rates of avian nest predation increase near habitat edges, more than half of 54 case studies reviewed by Lahti (Reference Lahti2001) failed to support a negative relationship between nest predation and distance from the forest edge. In some of these studies, putative edge effects may have been concealed due to inappropriate spatial or temporal scales of the study (Paton Reference Paton1994, Stephens et al. Reference Stephens, Koons, Rotella and Willey2003), insufficient statistical power (Lewis Reference Lewis2004), or the exclusive use of artificial nests which may mask patterns of natural nest predation (Haskell Reference Haskell1995a,Reference Haskellb, Zanette Reference Zanette2002, Burke et al. Reference Burke, Eliliott, Moore, Dunford, Nol, Phillips, Holmes and Freemark2004). However, well-designed studies also either failed to show significant edge effects (e.g. Hanski et al. Reference Hanski, Fenske and Niemi1996, Tewksbury et al. Reference Tewksbury, Hejl and Martin1998, Morse and Robinson Reference Morse and Robinson1999) or revealed heterogeneity in the strength or direction of the reported relationships (Hartley and Hunter Reference Hartley and Hunter1998, Lahti Reference Lahti2001, Chalfoun et al. Reference Chalfoun, Thompson and Ratnaswamy2002, Stephens et al. Reference Stephens, Koons, Rotella and Willey2003). Such heterogeneity may indicate that nesting species differ in their ability to cope with habitat change (e.g. through nest concealment or parental care; King et al. Reference King, Degraaf, Griffin and Maier1999, Martin et al. Reference Martin, Scott and Menge2000, Yasue and Dearden Reference Yasue and Dearden2006, but see Fontaine et al. Reference Fontaine, Martel, Markland, Niklison, Decker and Martin2007), or alternatively, that nest predators differ in their responses to habitat change (Chalfoun et al. Reference Chalfoun, Thompson and Ratnaswamy2002). Some predator species may be typically associated with edge and matrix habitat, and their numbers can be expected to increase when both edge-to-area and matrix-to-habitat ratios increase under increasing habitat fragmentation (Gates and Gysel Reference Gates and Gysel1978, Marzluff and Ewing Reference Marzluff and Ewing2001). In contrast, predator species restricted to pristine environments may be adversely affected by habitat degradation and fragmentation. Both in temperate (Andrén Reference Andrén1992, Nour et al. Reference Nour, Matthysen and Dhondt1993, Hannon and Cotterill Reference Hannon and Cotterill1998) and tropical forest communities (Gibbs Reference Gibbs1991, Telleria and Diaz Reference Telleria and Diaz1995), bird nests in the interior of forests are mainly predated by small mammals, while avian predators seem to be concentrated at forest edges. As a consequence, the strength and direction of edge effects on nest predation may be affected by the composition of predator communities, which in turn may vary with habitat type, landscape structure and geographic location (Tewksbury et al. Reference Tewksbury, Hejl and Martin1998, Chalfoun et al. Reference Chalfoun, Thompson and Ratnaswamy2002).
In East African forests, avian nest predation is mainly attributed to (small) mammals, rather than to birds (Carlson and Hartman Reference Carlson and Hartman2001, Githiru et al. Reference Githiru, Lens and Cresswell2005, Hanson et al. Reference Hanson, Newmark and Stanley2007). In the absence of important avian nest predators typically associated with edges, rates of nest predation can either be expected to show no edge effect or an inverse one, i.e. stronger predation with increasing distances from forest edges. We here test this hypothesis with the use of artificial ground nests baited with plasticine eggs. While the use of artificial nests is strongly debated (Zanette Reference Zanette2002, Burke et al. Reference Burke, Eliliott, Moore, Dunford, Nol, Phillips, Holmes and Freemark2004, Robinson et al. Reference Robinson, Styrsky and Brawn2005) because predators may be differentially attracted to natural and artificial nests (Montgomerie and Weatherhead Reference Montgomerie and Weatherhead1988, Willebrand and Marcström Reference Willebrand and Marcström1988, Martin et al. Reference Martin, Scott and Menge2000) or because quail or chicken eggs used in artificial nest experiments are too large to handle for small predators (Haskell Reference Haskell1995a,Reference Haskellb, Hanson et al. Reference Hanson, Newmark and Stanley2007), it also offers important advantages. First, confounding effects of laying date and microhabitat use, both of which were shown to affect predation rates on natural nests in our study area (Spanhove et al. Reference Spanhove, Lehouck, Boets and Lens2009), can be accounted for. Second, experiments with artificial nests can circumvent the problem of inadequate statistical power resulting from small samples of natural nests. The use of plasticine eggs has the additional advantage to reveal predator identity without intensive nest searching or the use of expensive optical equipment. However, results should be interpreted with caution as nest predation by small mammals may be overestimated (Pärt and Wretenberg Reference Pärt and Wretenberg2002). Based on these considerations and the fact that observed depredation on natural White-starred Robins Pogonocichla stellata nests (Spanhove et al. Reference Spanhove, Lehouck, Boets and Lens2009) corresponded well with that on a set of artificial nests in the same study area (Githiru et al. Reference Githiru, Lens and Cresswell2005), we here address the question if, and to what extent, spatial variation in artificial nest predation rates supports the presence of an inverse edge effect for a ground-nesting forest species.
Material and Methods
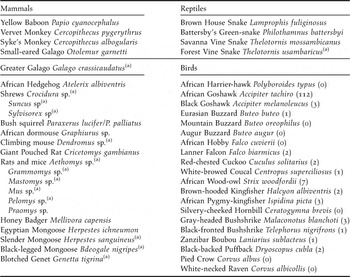
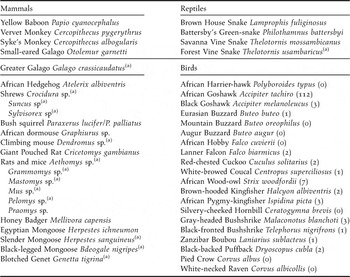
The Taita Hills of South-East Kenya (3°20’S 38°15’E) form the northernmost extension of the ancient Eastern Arc Mountains and are part of the Eastern Afromontane biodiversity hotspot (Mittermeier et al. Reference Mittermeier, Gil, Hoffman, Pilgrim, Brooks, Mittermeier, Lamoreux and da Fonseca2005, Burgess et al. Reference Burgess, Butynski, Cordeiro, Doggart, Fjeldså, Howell, Kilahama, Loader, Lovett, Mbilinyi, Menegon, Moyer, Nashanda, Perkin, Rovero, Stanley and Stuart2007). Within the study area, few fragments of (degraded) indigenous cloud forest persist in a landscape which is dominated by dry shrubland and small subsistence farmland (Beentje Reference Beentje1988). Table 1 lists all putative predators of bird eggs and nestlings in the Taita forest remnants, based on 12 years of mist-net captures (1996–2008), five years of behavioural observations (2003–2008) and literature data (Beentje Reference Beentje1988, Malonza Reference Malonza and Bytebier2001, Oguge Reference Oguge and Bytebier2001, R. Odhiambo unpubl. data, K. Malonza and G. J. Measey unpubl. data). We studied artificial nest predation rates in the two largest forest fragments located within the most heavily fragmented mountain isolate (Dabida) of the Taita Hills, where less than 2% of indigenous forest cover remains (Figure 1): Ngangao (133 ha indigenous forest/161 ha when including exotic plantations surrounding the indigenous forest) and Chawia (90 ha/95ha) (P. Pellikka unpubl. data).

Figure 1. Map of the Taita study area above the main forest-shrubland boundary (alt. 1,200 m). Black: indigenous forest fragments; dark grey: exotic plantations; light grey: woodland and isolated tree patches; white: settlements and small-holder cultivation plots (mainly maize, cabbages, beans and bananas). Based on aerial photographs (P. Pellikka, unpublished data).
Table 1. Putative nest predators in the indigenous forest fragments of the Taita Hills, south-east Kenya. For avian predators, numbers captured since 1996 are given between brackets (total number of captures was 20,701).
(a) presence in Taita Hills inferred from literature only (see text).
Between 18 January and 9 February 2007, i.e. late breeding season, a total of 14 (Ngangao) and 15 (Chawia) transects were established, each measuring 170 m, located perpendicularly to the forest edge and at least 100 m apart. Each transect started at a distinct edge between indigenous forest and agricultural land (‘cantilevered’ type sensu Murcia Reference Murcia1995), and ran through homogenous indigenous forest, i.e. avoiding exotic plantations and large tree-fall gaps. A total of 470 dome-shaped finch-nests (‘Birdie’ Art n° 100497, Flamingo, Herentals, Belgium) resembling those of ground nesting White-starred Robins and Yellow-throated Woodland-warblers Phylloscopus ruficapillus, were placed at ground level and concealed with leaves and branches in locations similar to those of natural nests. Each nest was baited with two plasticine eggs that were similar in size and shape to White-starred Robin eggs. To reduce the level of autocorrelation, nests were placed at 30 m intervals along each transect and were shifted twice (after six days of exposure) to cover all 10 m intervals in between. To reduce human scent, latex gloves were used during all manipulations of nests and eggs. Prior to formal hypothesis testing, we verified whether predation rates on nests at 10 m distance were statistically independent by comparing observed versus expected nests with 0, 1 and 2 predated neighbouring nests (G2 = 0.29, P = 0.86; expected numbers based on average predation rate per transect) (details in Hannon and Cotterill Reference Hannon and Cotterill1998). Within a radius of 5 m around each nest, we scored the presence of exotic trees and the structure of understorey and canopy (canopy: ‘open’ (< 50%) or ‘dense’ (> 50%) cover above 5 m; ‘gap’ if a gap of at least 10 m2 was present in this layer; understorey: ‘open’ (< 25%) or ‘dense’ (> 25%) cover lower than 1 m). The canopy was more open, the understory was denser and exotic trees were more abundant around artificial nests closer to the fragment edges (General Linear Models, all P < 0.05). After six days of exposure, each nest was checked for signs of predation. If tooth marks were present, eggs were collected and imprints were compared with mammalian skulls of species known to occur in the Taita Hills at the National Museums of Kenya (Nairobi, Kenya) and the Royal Museum of Central Africa (Tervuren, Belgium).
Predation rates were analysed with Generalized Linear Mixed Models in SAS v. 9.1.3 (PROC GLIMMIX, SAS Institute 2002–2003). Predation (1 or 0 after six days of exposure) was modelled as binomial distributed dependent variable; distance to the forest edge, fragment and/or habitat structure as fixed effects, and transect as random effect. Since complex models with random effects cannot reliably be compared with information criteria based on pseudo-likelihoods (SAS Institute 2008), we applied a classic stepwise forward procedure (critical P-value 0.05) for model selection. In models with random effects, denominator degrees of freedom were computed following Kenward and Roger (Reference Kenward and Roger1997). In all analyses, the square root of the distance between the nest and the forest edge was used to account for disproportional changes in predation and environmental parameters closer to the edges (Paton Reference Paton1994, Batáry and Báldi Reference Batáry and Báldi2004). In a simplified model without random effects, this measure proved a much better predictor than untransformed distances based on a small-sample Akaike Information Criterion (Hurvich and Tsai Reference Hurvich and Tsai1989, Burnham and Anderson Reference Burnham and Anderson2002; ΔAICc = 8.6).
Results
Avian nest predators were only rarely encountered in the Taita forests. Of a total of 20,701 birds trapped with mist-nests between 1996 and 2008, only 140 individuals (0.7%) were considered as potential nest predators (Table 1). Other potential avian predators such as African Harrier-hawk Polyboroides typus and Silvery-cheeked Hornbill Ceratogymna brevis were regularly observed in the intervening landscape matrix or forest canopy, but never in the forest understorey.
A total of 383 nests (81.5%) were predated after six days’ exposure trials. In Chawia, predation rates were significantly higher in the forest interior than near the forest edge (t = −4.69, dt = 466, P < 0.001). However, no such trend was apparent in Ngangao (t = −0.38, dt = 466, P = 0.70; Figure 2). In both fragments, predation increased with increasing density of canopy (F 2,466 = 3.55, P = 0.029) and understory vegetation (F 1,467 = 8.52, P = 0.0037). When applying a forward selection procedure, distance to the forest edge remained significant when accounting for effects of vegetation structure (Figure 2 and Table 2).

Figure 2. Inverse edge effect on artificial nest predation in Chawia (CH), but not in Ngangao (NG). Predation was higher in dense understorey (dense) than in open understorey (open) in both fragments. Lines depict model estimates, symbols depict observed predation rates.
Table 2. Tests of fixed and random effects on predation of 470 artificial nests. Results from generalized linear mixed models are shown for all predation events combined (pooled) and for each predation type separately (eggs missing; nest destroyed; tooth marks).

(a) non-significant terms excluded from the final model.
n.s.: P > 0.05; *: P < 0.05; **: P < 0.01; ***: P < 0.001.
In 242 predation events (63%), eggs were removed from intact nests, while in 70 others (18.5%) nests were destroyed, most likely by larger predators such as monkeys, galagos, carnivores, squirrels, pouch rats or hornbills. In three of these nests, eggs showed imprints of the Giant Pouched Rat Cricetomys gambianus. As judged from tooth marks on eggs in intact nests, small rodents and shrews accounted for predation of the remaining 71 nests (18.5%). In 57 of these, tooth marks could be assigned to mice (e.g. Praomys sp., Mus sp., Mastomys sp. or Grammomys sp.), while seven clutches showed imprints of a shrew (probably Crocidura olivieri) and four clutches showed imprints of a dormouse (probably Graphiurus microtis).
The proportion of nests assigned to either type of predation significantly different between both fragments (![]() = 49.1, P < 0.0001, Figure 3). In Chawia, eggs were more often removed from intact nests, while in Ngangao, nests were either destroyed or showed eggs with tooth marks more often (Figure 3). The proportion of nests that were either destroyed or contained eggs with imprints, did not vary in relation to vegetation structure or distance to the edge (Table 2). In contrast, the proportion of nests with missing eggs increased with density of the understorey and distance to the forest edge in Chawia, but not in Ngangao (Table 2, Figure 3).
= 49.1, P < 0.0001, Figure 3). In Chawia, eggs were more often removed from intact nests, while in Ngangao, nests were either destroyed or showed eggs with tooth marks more often (Figure 3). The proportion of nests that were either destroyed or contained eggs with imprints, did not vary in relation to vegetation structure or distance to the edge (Table 2). In contrast, the proportion of nests with missing eggs increased with density of the understorey and distance to the forest edge in Chawia, but not in Ngangao (Table 2, Figure 3).

Figure 3. Type of predation on 470 artificial nests in (a) Chawia and (b) Ngangao. Dark grey: intact nest with eggs missing; light grey: nest removed or destroyed; black: intact nest with eggs with tooth marks.
Discussion
In many regions, avian nests are believed to be prone to elevated predation levels near forest edges compared to the interior of pristine forests (Hartley and Hunter Reference Hartley and Hunter1998, Lahti Reference Lahti2001, Chalfoun et al. Reference Chalfoun, Thompson and Ratnaswamy2002, Stephens et al. Reference Stephens, Koons, Rotella and Willey2003). Such edge effect on nest predation was not supported by our artificial nest experiment in two indigenous forest fragments. Instead, a significant inverse edge-effect, i.e. higher predation rates in the forest interior, was evident in Chawia, but not in Ngangao. Both fragments only lie 11 km apart and are similar in general appearance, although fragment Chawia has a more dense herb layer and lacks typical highland canopy species such as Podocarpus spp. and Ocotea spp, possibly due to its lower elevation. Despite being smaller, Chawia (90 ha) has relative more forest interior, but is more isolated from other forest patches than Ngangao (133 ha). Both fragments are surrounded by densely populated subsistence farmlands, although levels of human disturbance (mainly related to firewood collection) and densities of small rodents are higher in fragment Chawia (Oguge Reference Oguge and Bytebier2001, R. Odhiambo unpubl. data). While these, and probably other differences between Chawia and Ngangao may have contributed to the variation in edge effect on nest predation, lack of replicate forest fragments prevents us from drawing solid conclusions on the effects of fragment size, isolation and/or vegetation structure separately. Moreover, an earlier study on natural White-starred Robin nests revealed an inverse edge effect on nest predation in Ngangao as well (Spanhove et al. Reference Spanhove, Lehouck, Boets and Lens2009).
Heterogeneous conclusions drawn from studies on natural and artificial nests have fuelled criticism of the use of the latter in ecological research (Willebrand and Marcström Reference Willebrand and Marcström1988, Haskell Reference Haskell1995a,Reference Haskellb, Zanette Reference Zanette2002, Burke et al. Reference Burke, Eliliott, Moore, Dunford, Nol, Phillips, Holmes and Freemark2004). In our study area, artificial nests were more heavily predated than natural ones (Spanhove et al. Reference Spanhove, Lehouck, Boets and Lens2009), even when accounting for effects of laying date. While such a difference may be due to differential predator attraction to artificial and natural nests, it may also reflect shifts in predator composition during the course of a breeding season, or low statistical power due to small samples of natural nests. While absolute predation rates differed between artificial and natural nests, relative predation patterns on these two nest types were largely comparable. First, the distribution of predation events across three major types (nest destroyed, eggs missing, tooth marks) did not differ between 470 artificial nests (this study) and 51 natural ones (T. Spanhove unpubl. data; ![]() = 1.44, P = 0.44). Second, for both nest types, predation rates were higher in Chawia. Third, both nest types documented an inverse edge effect, a pattern rarely documented in other regions (Hartley and Hunter Reference Hartley and Hunter1998, Lahti Reference Lahti2001, Stephens et al. Reference Stephens, Koons, Rotella and Willey2003).
= 1.44, P = 0.44). Second, for both nest types, predation rates were higher in Chawia. Third, both nest types documented an inverse edge effect, a pattern rarely documented in other regions (Hartley and Hunter Reference Hartley and Hunter1998, Lahti Reference Lahti2001, Stephens et al. Reference Stephens, Koons, Rotella and Willey2003).
The lack of positive edge effects in the Taita Hills (this study, Githiru et al. Reference Githiru, Lens and Cresswell2005, Spanhove et al. Reference Spanhove, Lehouck, Boets and Lens2009) and other East African forests (Carlson and Hartman Reference Carlson and Hartman2001, Maina and Jackson Reference Maina and Jackson2003, Hanson et al. Reference Hanson, Newmark and Stanley2007) may be related to the absence of avian predators that are closely associated with agricultural fields and forest edges. In regions where avian predators dominate the nest predator community, predation levels are often higher at forest edges (Nearctic: Hannon and Cotterill Reference Hannon and Cotterill1998, Tewksbury et al. Reference Tewksbury, Garner, Garner, Lloyd, Saab and Martin2006; Palearctic: Nour et al. Reference Nour, Matthysen and Dhondt1993, Mazgajski and Rejt Reference Mazgajski and Rejt2005; Neotropic: Gibbs Reference Gibbs1991, Telleria and Diaz, Reference Telleria and Diaz1995; Indomalayan: Posa et al. Reference Posa, Sodhi and Koh2007, but see Pangau-Adam et al. Reference Pangau-Adam, Waltert and Muhlenberg2006; Australasian: Hausmann et al. Reference Hausmann, Catterall and Piper2005). Although the absence of avian predators may result in a lack of positive edge effects, it cannot explain the high predation levels in the forest interior. Such inverse edge effect may well be attributed to other predator species. However, the specific identity of nest predators cannot reliably be inferred from the remains of nests and eggs, especially when eggs are partly or completely removed from the nests (Larivière Reference Larivière1999). As eggs were removed from the majority of predated nests in this study, inferences on (shifts in) predator communities should be interpreted with caution. When combining all available evidence, however, larger predators such as monkeys and pouch rats can cautiously be ruled out as egg removers, since they would likely damage the small and fragile nest openings, while avian nest predators can be ruled out based on their documented rarity in the Taita forests. Eggs are commonly removed by snakes (Robinson and Robinson Reference Robinson and Robinson2001, Pärt and Wretenberg Reference Pärt and Wretenberg2002, Weatherhead and Blouin-Demers Reference Weatherhead and Blouin-Demers2004), although these were rarely recorded in the study area (Malonza Reference Malonza and Bytebier2001). In contrast, small mammals such as mice and shrews, which can also remove entire clutches or consume them on the scene (Larivière Reference Larivière1999) are abundant, particularly so in forest fragment Chawia (Oguge Reference Oguge and Bytebier2001, R. Odhiambo unpubl. data). Although small mammals are most likely responsible for the majority of predation events on plasticine eggs, their role in natural nest predation remains vague (Pärt and Wretenberg Reference Pärt and Wretenberg2002).
Mortality due to nest predation is often regarded as a major driver of the population dynamics of small forest birds. For instance, high levels of nest predation in Chawia may underlie the poor recruitment, skewed sex ratios and lack of floaters in this fragment (Githiru and Lens Reference Githiru and Lens2006, Githiru et al. Reference Githiru, Lens, Bennun and Perrins2006), despite being the third largest fragment in the Taita Hills. To improve future population viability prospects for small forest birds, conservation plans need to take into account the processes underlying nest predation. For instance, if high predation rates would mainly reflect high rodent densities, (re)introduction and conservation of top predators may relieve forest birds from unnaturally high predation pressure (Crooks and Soulé Reference Crooks and Soule1999, Maina and Jackson Reference Maina and Jackson2003). Alternatively, if higher predation rates would mainly reflect better thermal conditions for snakes, e.g. due to elevational and structural properties of the forest fragments (Malonza Reference Malonza and Bytebier2001), projected climate changes may reinforce these effects, independently of local habitat restoration programmes. Effective conservation actions therefore remain in need of small-scale, high-resolution studies, such as this one, to better understand the ecological processes underlying spatio-temporal variation in nest predation.
Acknowledgements
We are grateful to the Kenyan government for approving this research project under MOEST Ref. N° 13/001/33C 294/2. The staff of the Mammalogy and Osteology Departments of the National Museums of Kenya and the Royal Museum for Central Africa granted access to their collections. S. Sokol assisted with fieldwork, K. Geurts with identification of the tooth marks, and F. Hendrickx with statistical analysis. R. O. Odhiambo, K. Malonza and G. J. Measey provided unpublished information on the occurrence of small mammals and reptiles in the Taita Hills; P. Pellikka kindly supplied a map of the study area. Two anonymous reviewers provided helpful comments on earlier versions of the manuscript. This study was supported by projects G.0210.04 and G.0055.08 of Research Foundation - Flanders (FWO), the Science faculty of Ghent University, and VLIR-UOS. TS and VL are research assistants of FWO.