Introduction
The Taita Hills of south-eastern Kenya are an ancient mountain massif forming the northernmost reach of the Eastern Arc Mountains range, one of 34 global biodiversity hotspots (Mittermeier et al. Reference Mittermeier, Robles Gil, Hoffmann, Pilgrim, Brooks, Mittermeier, Lamoreux and da Fonseca2004). The tiny forest remnants of the Taita Hills boast a remarkable amount of endemic biodiversity, including plants, vertebrates and invertebrates (Burgess et al. Reference Burgess, Butynski, Cordeiro, Doggart, Fjeldsa, Howell, Kilahama, Loader, Lovett, Mbilinyi, Menegon, Moyer, Nashanda, Perkin, Rovero, Stanley and Stuart2007), but the survival of these species is threatened by a rapidly expanding human population. Currently, natural forest in the hills is restricted to 12 small fragments embedded in a landscape dominated by smallholder agriculture and villages, where the human population is approximately 250,000 and density reaches 1,400 individuals km−2 (BirdLife International 2008a).
Two species of birds, Taita Thrush Turdus helleri and Taita Apalis Apalis fuscigularis are endemic to the Taita, while a third one (Taita White-eye Zosterops silvanus, also found on nearby Mount Kasigau) has been treated as a full species or a subspecies of the more broadly-ranging Montane White-eye Zosterops poliogastrus (Collar et al. Reference Collar, Crosby and Stattersfield1994, Stattersfield et al. Reference Stattersfield, Capper and Dutson2000). All these taxa are threatened by degradation of their extremely restricted ranges, but while more work has been done on the thrush (Lens et al. Reference Lens, Galbusera, Brooks, Waiyaki and Schenck1998, Reference Lens, Van Dongen and Matthysen2002a, Lens and Eggermont Reference Lens and Eggermont2008, Galbusera et al. Reference Galbusera, Lens, Schenck, Waiyaki and Matthysen2000) and on the white-eye (Mulwa et al. Reference Mulwa, Bennun, Ogol and Lens2007), very limited information is currently available on the apalis. Apalis fuscigularis is listed as ’Critically Endangered’ in the IUCN Red List (BirdLife International 2008b), but no accurate assessment of its population size and ecology has ever been published.
In this paper, we report the results of the first population survey of Taita Apalis, and analyse preliminary data on its habitat use. This survey will provide a baseline to evaluate future population trends and will guide conservation efforts that urgently need to be stepped up in order to avert the disappearance of this species.
Study area
The Taita Hills (38°20′E 3°25′S) are an isolated massif approximately 20 × 20 km in size, rising to more than 2,200 m a.s.l. from the surrounding dry plains at 900 m. Originally, most of the hills were probably covered with trees, but forest is now restricted to the highest peaks and steepest slopes surrounded by a dense matrix of human settlements. The total amount of closed-canopy natural forest on the hills is about 400–600 ha, subdivided into 12 fragments with a range in size of 1–220 ha (Rogo and Oguge Reference Rogo and Oguge2000, Pellikka et al. Reference Pellikka, Lötjönen, Siljander and Lens2009). Both the physical structure and the flora of the fragments are influenced by human disturbance, which is less pronounced in the larger fragments. Logging was intense in the area up to the 1970s, and most of the commercially valuable timber species (e.g. Ocotea usambarensis, Podocarpus spp.) have been removed, resulting in sometimes discontinuous canopy cover and increased dominance by early successional trees such as Tabernaemontana stapfiana (Wilder et al. Reference Wilder, Brooks and Lens2000, Bytebier Reference Bytebier2001, Chege and Bytebier Reference Chege and Bytebier2005). Between the 1950s and 1970s, the Kenya Forest Department (now Kenya Forest Service) created a number of plantations of non-indigenous trees (Pinus patula, Cupressus lusitanica and Acacia mearnsii) inside the forest fragments, aiming to reduce soil erosion and provide an alternative source of wood for the human population. Aerial photographs taken in 1955 suggest that approximately 50% of the area of natural forest has been converted to tree plantations in recent decades (Pellikka et al. Reference Pellikka, Lötjönen, Siljander and Lens2009).
This study was carried out in all the forest fragments (Chawia, Ngangao, Vuria and Fururu, Fig. 1) where Taita Apalis was reported in the past (Brooks et al. Reference Brooks, Lens, Barnes, Barnes, Kageche Kihuria and Wilder1998a). To clarify the habitat preferences of the species we also sampled habitat structure in these four fragments, plus two others (Mbololo, Yale) where the apalis has never been reported but another ’Critically Endangered’ endemic bird, Taita Thrush Turdus helleri occurs (Brooks et al. Reference Brooks, Lens, Barnes, Barnes, Kageche Kihuria and Wilder1998a). A description of the studied fragments follows; areas (which we used in population size estimates) are taken from Pellikka et al. (Reference Pellikka, Lötjönen, Siljander and Lens2009) or, for fragments not covered in that study, from our own GPS readings recorded during the fieldwork.
• Ngangao: the second-largest fragment (altitude: 1,750–2,000 m) with a size of 206 ha, of which 120 ha are natural forest and the rest exotic plantations and rocky outcrops (Pellikka et al. Reference Pellikka, Lötjönen, Siljander and Lens2009). Although pit-sawing was carried out in this fragment in the past (LB pers. obs.), little human disturbance occurs now, as shown by a well-structured tree abundance-diameter curve and rich flora (Wilder et al. Reference Wilder, Brooks and Lens2000). Ngangao is split into two sections (Ngangao North: 64 ha and Ngangao South: 56 ha) by a large rocky outcrop. These two sections, although connected by a corridor of forest, are considered separately in this paper, because we found significant differences in the abundance of Taita Apalis between them.
• Chawia: a medium-sized fragment (altitude: 1,650–1,670 m), with 86 ha of indigenous forest (Pellikka et al. Reference Pellikka, Lötjönen, Siljander and Lens2009). It is extensively disturbed, as shown by the discontinuous canopy dominated by Tabernaemontana stapfiana and Albizia gummifera, two early-successional species. Until recently, wood cutting for fuel and grazing of cows was practiced in this fragment, resulting in a marked reduction in small and mid-sized trees (< 30 cm diameter; Wilder et al. Reference Wilder, Brooks and Lens2000).
• Vuria: this fragment is located on a steep ridge (altitude: 1,900–2,200 m) and suffered extensive deforestation and fires in the past. Woodcutting and collection of forest products by resident people still widely occurs in this area. The remaining closed-canopy forest is about 1 ha, less that 1% of its former size (Beentje Reference Beentje1988). However, in Vuria we found Taita Apalis also outside of true forest, in the extensive (51 ha) montane scrubland with scattered trees that surrounds the forest remnants.
• Fururu: a disturbed fragment (altitude: 1,700–1,800 m), with a total area of 62 ha, of which only 8 ha are natural forest, and the rest exotic tree plantations (Pellikka et al. Reference Pellikka, Lötjönen, Siljander and Lens2009). Taita Apalis occurred in this fragment until recent times (Brooks et al. Reference Brooks, Lens, De Meyer, Waiyaki and Wilder1998b).
• Yale: a fragment at about 1,950 m altitude, with 16 ha of natural forest (Pellikka et al. Reference Pellikka, Lötjönen, Siljander and Lens2009) and relatively good arboreal vegetation (Chege and Bytebier Reference Chege and Bytebier2005). Even though Taita Apalis has never been reported, Taita Thrush occurs here, suggesting that other locally endemic species might also be present
• Mbololo: the largest forest fragment (220 ha), with the best preserved vegetation and richest flora, dominated by primary forest trees such as Aningeria adolfi-frederici and Strombosia schlefferi (Wilder et al. Reference Wilder, Brooks and Lens2000) at 1,400–1,800 m. Mbololo is the most important stronghold for Taita Thrush (Lens et al. Reference Lens, Van Dongen and Matthysen2002a), but Taita Apalis has never been observed.
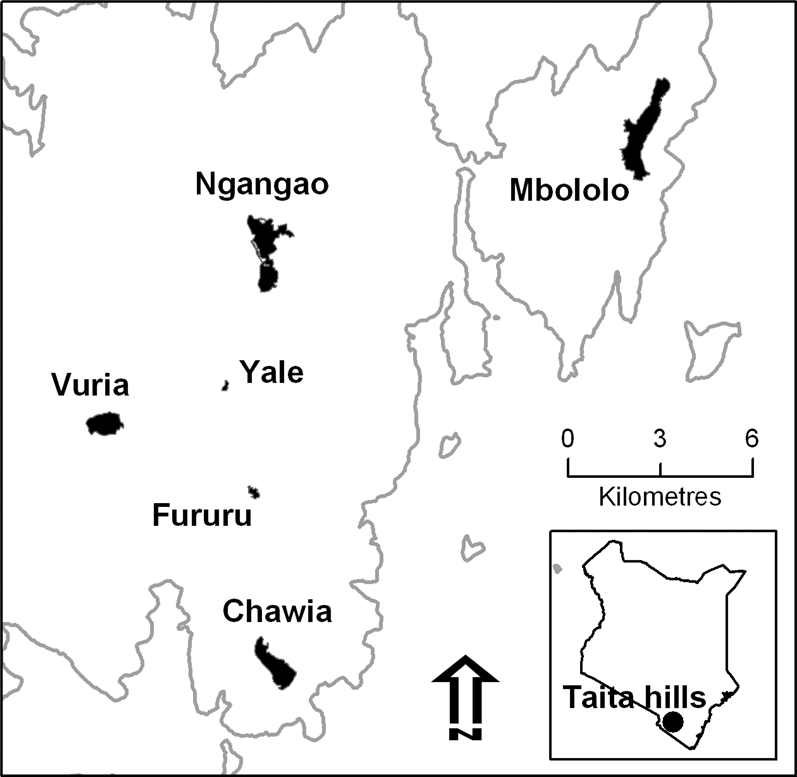
Figure 1. Map of the Taita Hills and of the forest fragments where this survey was carried out. The grey line marks the 1,000 m contour.
Field methods
Bird counts
We estimated population density in Ngangao, Chawia and Vuria with unlimited distance point counts carried out in two rounds between 24 February and 6 June 2001 (round 1) and between 29 June and 15 September 2001 (round 2). Sample points were located in natural habitats (i.e. we excluded exotic tree plantations) and formed a grid with 75 m-sized cells that covered the entire area of the sampled fragments. During each of the two rounds of counts, we sampled points along alternate lines of the grid (i.e. lines sampled during each round were laterally spaced by 150 m, and points were placed at 75 m intervals along each line). Thus, half of the grid in each fragment was sampled in each round, and each fragment was covered with approximately equal sampling effort in the two rounds. We used compass and measuring tape to navigate between points, and we surveyed 412 points (Chawia: 130, Ngangao North: 102, Ngangao South: 85, Vuria: 95). Survey effort ranged between 1.5 (Chawia) and 1.8 (Vuria) points ha-1 due to inaccessibility of a few points located on very steep slopes. The survey team consisted of three observers in order to maximise the number of records of this rare and secretive species. Observers counted birds for 20 minutes, recording both visual and aural signs of presence of the target species. We visually estimated distances from the census point to the birds. We also recorded the time elapsed from the start of the count to each detection. All counts were carried out in clear weather conditions, and observers practiced estimating distances before the start of the survey. Since preliminary observations suggested that vocal activity of the species did not vary much through time, counts were carried out at all times of the day, except in the early hours (06h00–08h00) when intense vocal activity of other species made detection of apalis calls more difficult.
Habitat description
We recorded 11 habitat descriptors to assess habitat use and differences between occupied and unoccupied forest fragments. In Chawia, Ngangao and Vuria, data were collected inside 10 m-radius plots centred on the sample points where the bird count was carried out. Due to practical constraints (e.g. steep slopes or meteorological conditions), the number of points where habitat data were recorded (383) is lower than the total number of points where the bird counts were carried out (412). We also recorded habitat data at 183 additional points in Mbololo, Yale and Fururu (Taita Apalis absent). In these fragments, habitat data were recorded at 75 m intervals along randomly located transects running through the fragment. Eight structural habitat descriptors were recorded: the number of tree stems in four classes of diameter at breast height (< 10 cm, 10–20 cm, 20–30 cm, >30 cm), the number of cut woody stems (an estimator of intensity of human activity), and visual estimates of percentage canopy cover, average canopy height and percentage of trees covered by climbers. Three floristic habitat descriptors were also visually estimated: percentage cover of two gap-selecting heliophilous species (the bracken fern Pteridium aquilinum and the shrub Plectranthus barbatus) and percentage cover of one sciaphilous forest understory species, the shrub Dracaena laxissima.
Data analysis
Population estimates
To generate estimates of density and population size in each fragment, we used the software DISTANCE 5.0 (Thomas et al. Reference Thomas, Laake, Strindberg, Marques, Buckland, Borchers, Anderson, Burnham, Hedley, Pollard, Bishop and Marques2006). We plotted a frequency histogram of all observation distances, which suggested heaping (i.e. a tendency to round distances to certain preferred values); distances were therefore grouped into bands (0–16, 17–24, 25–40 and 41–60 m) in order to smooth the irregularities. We discarded 11 observations beyond 60 m (9% of total) to improve the fit of the detection functions. In order to verify if data collected in different fragments and separate census rounds could be pooled into a single detection function, we fitted preliminary detection functions to the observations recorded in each fragment and in the two census rounds, and compared values of the Akaike Information Criterion (AIC) for the global and the stratified data. Data pooling is acceptable if the sum of the AICs for the stratified data is higher than that of the pooled data (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001).
Census duration is a critical factor in the estimation of density by distance sampling. Short times can cause g(0), the detection function at zero distance, to be less than 1 due to individuals that escape detection despite being close to the observers (Bächler and Liechti Reference Bächler and Liechti2007). This produces negative bias in the estimates of population density. On the contrary, long count times bias estimates up due to double-counting and to individuals that move into the detection range during the count time. In order to assess optimal census duration, we grouped our observations into subsets of progressively increasing duration (6-8-10-15-20 minutes) and produced density estimates for each of these subsets. We also assessed if detection distances increased with time, which is an indication of movements of birds into the detection range during the count (Lee and Marsden Reference Lee and Marsden2008a).
As most (87%) of the observations were auditory and group size cannot be accurately estimated by aural detections (Hayward et al. Reference Hayward, Kepler and Scott1991), we generated a single estimate of group size and of its standard error (1.67 ± 0.16) using the 15 visual observations that were obtained during the survey (one in Chawia, five in Ngangao and nine in Vuria; range 1–3 individuals). All these groups were observed well (less than 15 m from the observers), and we found no difference between fragments or count rounds (Mann-Whitney test, P > 0.05 in all pairwise comparisons). We therefore assumed constant group size across sites and census rounds.
We used AIC to select the model that best fitted the distribution of observation distances. Models whose AIC differed by less than four units were considered acceptable and averaged (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001) using 999 bootstrap replications to generate, separately for each fragment, final estimates of density of groups of Taita Apalis as well as their log-normally distributed 95% confidence intervals (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). Density of individuals and total population size were calculated by multiplying estimated group size by estimates of group density (groups ha−1) produced by DISTANCE for each fragment. Confidence intervals for these calculated values were generated with the delta method (Seber Reference Seber1982).
As a final step, we tested if subpopulations of Taita Apalis located in Chawia, the two sections of Ngangao and Vuria had different density. However, our density estimates cannot be directly compared between forest fragments, because they are not statistically independent, as we assumed constant group size and detection function. Therefore, we used Z-tests to compare encounter rates, and we assume that different encounter rates in two fragments are an indication that those fragments also have different densities of the focal species.
Habitat use
In order to find if there was any difference in the habitats of occupied versus unoccupied fragments, we used univariate ANOVAs to compare the eleven habitat descriptors across all the forest fragments. For these analyses, counts were log-transformed and percentages arcsine-square-root transformed. As habitat descriptors are usually correlated and show complex patterns of variation that are not easily highlighted by univariate analyses, we further explored the variability in habitat structure using non-metric multidimensional scaling (NMDS: Legendre and Legendre Reference Legendre and Legendre1998) to draw a two-dimensional graphic representation of vegetation structure across study sites. NMDS is particularly suited to the analysis of ecological data, as it is a robust ordination technique making no assumptions on the distribution or the shape of the relationships between variables. NMDS produces an ordination of the sampling units (the plots where habitat structure was assessed) on an arbitrarily chosen number of axes correlated to the original variables, and can therefore summarise general patterns of variation within complex datasets.
We also compared the characteristics of the sample points where Taita Apalis was found with the points were the species was absent. This analysis was done only within the fragments where Taita Apalis was observed (Chawia, Ngangao and Vuria), because sites in unoccupied fragments may be empty for reasons not related to habitat characteristics (Lens et al. Reference Lens, Van Dongen, Norris, Githiru and Matthysen2002b). Since we recorded habitat descriptors in 10 m-radius plots centred on the census point, and most of the birds contacted during the counts were more than 10 m from the observers, there is a risk that habitat measurements did not reflect the habitat used by birds located far from the observers. Therefore, we first analysed the entire set of presence records of Taita Apalis, but then repeated the analyses with subsets including only records obtained at < 40 m, < 20 m and < 10 m (n = 118, 82, 54 and 13 observations respectively). We used a generalised linear model with binomial error and logit link to model the presence/absence. We reduced multicolinearity in the dataset by calculating pairwise correlations (Spearman’s Rho) between pairs of habitat variables and retaining only one habitat descriptor (the one with highest univariate fit to the Taita Apalis presence data as suggested by AIC) in each pair of variables with Rho ≥ 0.4. This resulted in a total of six habitat descriptors assessed (cover of Dracaena and Plectranthus, abundance of trees with diameter between 10 and 20 cm, canopy height, cover of climbers and number of cut stems). Interactions between factors as well as squared terms were not considered to avoid the risk of overfitting the model (Burnham and Anderson Reference Burnham and Anderson2002).
Spatial autocorrelation in the data was indicated by significant (P < 0.05) Moran’s I index (Moran Reference Moran1950), ranging between 0.22 and 0.32 up to 300 m around the sample points. Autocorrelation causes increased type I (false positive) error in statistical tests (Legendre and Legendre Reference Legendre and Legendre1998), therefore we controlled it by adding two additional factors in the models. The first (SITE) had levels corresponding to each of the four sites (Ngangao South and North, Vuria and Chawia) and represented variation in observation frequency across forest fragments. The second was an autoregressive term (Rangel et al. Reference Rangel, Diniz-Filho and Bini2006):
where ρ is the autoregression parameter and the matrix W contains weights wij calculated as an inverse function of geographical distances between couples of sample points. Autocorrelation can be caused by the response of the target species to spatially structured habitat features, which means that controlling spatial autocorrelation can lead to underestimating the environmental factors that control the distribution of a species (Dormann et al. Reference Dormann, McPherson, Araújo, Bivand, Bolliger, Carl, Davies, Hirzel, Jetz and Kissling2007). For this reason, besides spatially-corrected models (six habitat descriptors + site or autoregressive term), we also consider partial models including habitat factors alone.
Model selection was carried out using an information-theoretic approach (Burnham and Anderson Reference Burnham and Anderson2002). We tested all the possible combinations of variables, and all the models with AIC values within four units from the minimum were considered acceptable. We assessed model performance with Cohen’s kappa (Cohen Reference Cohen1960), an estimator of the agreement between observed and modelled data that controls for agreement occurring by chance alone. Values of kappa between 0 and 0.40 indicate slight to fair, 0.41–0.60 moderate, and values above 0.61 good model performance.
Results
Population estimates
We found Taita Apalis in only three fragments (Ngangao, Vuria and Chawia). In Ngangao and Chawia, the species only occurred in natural forest, but in Vuria it was observed mainly in the scrub-like vegetation with scattered remnant trees that forms most of the natural vegetation of this fragment. These three fragments were the object of the population survey, which yielded 129 records of the focal species, of which 118 were inside the 60 m truncation distance (Chawia: 130 points and 6 records; Ngangao North: 102 and 55; Ngangao South: 85 and 19; Vuria: 95 and 38).
As AIC values of a global detection function were > 4 units lower than summed AICs for the stratified data, we pooled all the observations across fragments and census rounds in a single detection function. A model with Hazard-rate key function and no adjustments scored the lowest AIC value, but two others (Half-normal + cosine and Uniform + polynomial) were also possible candidates (Δ AIC < 4; Fig. 2).

Figure 2. Histograms of the observations (scaled by detection distances) with fitted detection functions
Density estimates produced by all candidate models increased with count duration (Fig. 3). The longest count time (20 min) produced estimates more than twice larger than those produced by the shortest count time (6 min). However, all models estimated a global population of less than 1,000 individuals (Table 1).

Figure 3. Relationship between estimated density (± SE) of Apalis fuscigularis and duration of the count time (6-8-10-15-20 min). Points have been slightly staggered along the x-axis to improve readability.
Table 1. Estimates of density (individuals ha−1) and population size (number of individuals.) produced by averaging 999 bootstrap replicates of three detection functions (Hazard rate, Half-normal + cosine, Uniform + cosine) assuming group size = 1.67 ± 0.16SE.

Average detection distances (28.2 m, n = 129) did not increase with count duration (Least-squares regression, r 2 = 0.006, P = 0.43). Substantial numbers of detections at less than 20 m from the observers were obtained even towards the end of the 20-min count (Fig. 4).

Figure 4. Scatterplot of detection distances (left y-axis) and time elapsed from the beginning of the count. The solid line, whose units are on the right y-axis, shows the total number of observations accumulated during the 20-min count time in the entire set of 412 points surveyed in Ngangao, Chawia and Vuria
Encounter rates varied between fragments. Chawia had lower rates than all other fragments, and Ngangao North had higher encounter rates than Ngangao South for all count durations (Z-test, all P < 0.05). Vuria did not differ from Ngangao North, but had significantly higher encounter rates than Ngangao South at 10 and 20 min count durations (Z-test, P < 0.05).
Habitat use
There were highly significant differences between the mean values of all 11 habitat descriptors in the forest fragments (Table 2). In general, larger fragments (Mbololo, Ngangao north and south) had higher densities of trees in all diameter classes, lower number of cut woody stems, higher cover of Dracaena and lower of Plectranthus and Pteridium. However, Chawia, despite being a fairly large fragment (91 ha), with a discontinuous but tall canopy, scored the lowest densities of trees in the classes between 0 and 30 cm diameter.
Table 2. Mean values (± SE) of eleven habitat variables in seven forest fragments. Differences were tested with univariate Anovas. Letters mark homogeneous groups (P < 0.05) as recognized by post-hoc Tukey HSD tests

NMDS produced a two-dimensional ordination that captured a substantial proportion of the variability in the original data (cumulative r 2 between ordination distance and original data = 0.82). Axis 1 (x-axis in Fig. 5) represented a gradient from tall, continuous canopy with Dracaena undergrowth to low, discontinuous thicket with high human disturbance and dense Pteridium and Plectranthus. Axis 2 (y-axis in Fig. 5) was a gradient of decreasing tree density (high negative correlations with number of trees in all diameter classes). Figure 4 shows that the sample plots were mostly arranged along a line from bottom-left toward top-right, representing a gradient from dense, little disturbed to open, bushy and severely disturbed. Even though some differences between forest fragments are evident in Fig 5 (e.g. most plots in Vuria are in the top right and most of those in Chawia in the top left quarter of the plot), much overlap in the vegetation structure of different fragments is also evident.

Figure 5. NMDS ordination of 566 sample plots described by 11 habitat variables in the six forest fragments. Plots marked as “Apalis presence” (n = 82) are only those where the species was contacted at less than 40 m from the observer. Shown is also the 95% confidence ellipse of Apalis fuscigularis presence points.
Modelling of habitat use suggested a substantial amount of spatial structuring in the distribution of the observations, as models that included either the site factor or the autoregression term performed better than those containing only habitat variables, as suggested by lower AIC and higher Cohen’s kappa (Table 3). In general, models that included the autoregression term had slightly better performance than those with the site factor, except in the 10 m data subset, where performance of the two model structures was similar (Δ AIC < 2, Table 3). Models that included all the observations selected similar sets of variables to those obtained from subsets including only birds at 40, 20 or 10 m from the observer. The species showed a preference for sites with higher cover of climbers and, to a lesser extent, of Dracaena. When the site factor and the autoregression term were not included in the models, there was also a preference for denser tree cover, lower canopies and more dense cover of Plectranthus, but the predictive power of habitat descriptors alone was poor (Cohen’s kappa always < 0.15, Table 3). The absence of strong preferences for any of the habitat descriptors is confirmed by the broad scattering of Taita Apalis presence points in the entire NMDS ordination plot (Fig. 5).
Table 3. Results of logistic model of habitat selection of Apalis fuscigularis. The column “data in the model” shows what subset of observations was used in the model (all the data, or only birds within 40, 20 or 10 m from the observer). Model structure specifies whether either a spatial autoregressive term or a site factor was included in the model with the six habitat descriptors. “Number of models” column shows how many models were found within four AIC units the “best” model whose AIC and Cohen’s kappa statistics are given in the two rightmost columns of the table. All the models included in this table were significant at P < 0.01. Figures under the variable names are percentages of the number of models where that variable was included (e.g. 100 means that variable was found in all the models with AIC within four units from the minimum). Plus or minus symbols show the direction of the relationship between that variable and the presence of Apalis fuscigularis. Figures in bold show the factors included in the best model as indicated by the lowest AIC for that model structure.

Discussion
This survey reports the first estimates of density and population size for Taita Apalis. Our work was complicated by the characteristics of the focal species, a small bird that lives in dense vegetation and can be difficult to detect when it is silent. The choice of the detection function affected the estimates, as AIC values did not differ enough to allow secure distinction among models. We suggest that, in order to achieve better distinction between candidate functions, future surveys should aim to collect larger sample sizes and more accurate measurements of sighting distances (e.g. by using laser rangefinders, which were not available to us). Density estimates were also affected by count duration, as the longest count time (20 min) generated estimates twice as large as the shortest time (6 min). Upward bias in density estimates can be caused by double counting (multiple detections of the same individuals) or by birds entering into the detection range during count time. Double counting usually occurs with species that are active, sing infrequently, and move tens of meters between songs (Cimprich Reference Cimprich2009). This applies only in part to Taita Apalis, which in our experience does sing infrequently but also spends long times foraging on the same tree and rarely moves long distances between one foraging location and the next. Moreover, birds moving into the detection range should cause increasing detection distances towards the end of the count time (Lee and Marsden Reference Lee and Marsden2008a), which was not the case in our survey. This suggests that Taita Apalis did not move much during the count time. Thus, bias caused by movement and double counting was probably not major. Instead, we suggest that estimates at short count times were biased low due to incomplete detection of silent birds close to the observer. As count duration increased, these individuals were detected when they sang. Indeed, in our survey, birds close to the observers were observed even at the end of the 20 min count time, suggesting that, at the shortest count durations, probability of detection at zero distance were lower than 1, violating an assumption of distance sampling (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). In summary, we suggest that estimates generated by 6 and 8 minute count times are biased down. Future surveys should adopt long count durations (no less than 10 min) and observers should take care to avoid missing silent birds immediately around them.
Two important points emerge from our estimates. First, even in the most optimistic case, the global population of the species is less than 1,000 individuals. Second, there is a large variation between different fragments, with Ngangao South and Chawia supporting densities three and 10 times lower than Ngangao North respectively. Ngangao North alone supports approximately 50% of the global population of Taita Apalis in an area of 64 ha of forest, enhancing the threats to the species, whose global population could be instantly halved by a single catastrophic event, such as a large fire.
Understanding which factors cause the density variations across forest fragments is a conservation priority. First of all, the abundance of Taita Apalis could be affected by competition from the closely related Black-headed Apalis Apalis melanocephala. However, competition between these two species does not seem likely, as Black-headed Apalis is a canopy dweller (Del Hoyo et al. Reference Del Hoyo, Elliot and Christie2006), while Taita Apalis lives in the shrubs and low trees (BirdLife International 2008b), thus minimising the chances of competition between the two species. Another factor potentially affecting the abundance of Taita Apalis is habitat structure, as we found that the species prefers sites with greater cover of climbers, perhaps because dense leafy clumps provide a favourable foraging microhabitat. However, the selection for dense climbers contrasts with the low bird densities found at Chawia, where climber cover is the highest among the studied fragments. The low densities at Chawia might be caused by reduced tree stem density in this fragment, but our habitat selection models only showed a marginal positive preference of Taita Apalis for sites with many trees. Moreover, habitat descriptors, when considered alone, had a low power to predict the presence of the species (Table 3). In conclusion, our data do not suggest that significant variation in the abundance of Taita Apalis across forest fragments can be explained by habitat structure or floristics. As the range of the 11 habitat descriptors that we analysed was quite broad and included geographical, structural and floristic variables, as recommended by Lee and Marsden (Reference Lee and Marsden2008b), it seems unlikely that our analysis could have missed factors of such an importance that they can cause a 10-fold abundance variation, as observed between Chawia and Ngangao North.
We also found a significant amount of spatial autocorrelation in the distribution of the apalis, and the addition of an autoregression term improved the performance of logistic models, indicating that the distribution of Taita Apalis was spatially structured. Moreover, adding a site factor to the models improved their performance almost as much as the autoregression term, suggesting that much of the spatial structure in the distribution of Taita Apalis was due to between- rather than within-fragment variation. This spatial structure might be generated by social organisation or conspecific attraction (Stamps Reference Stamps1988, Morris et al. Reference Morris, Wiser and Klepetka1992), but this is unlikely as most Apalis warblers live in pairs that occupy independent territories without social organisation above the pair or family level (Del Hoyo et al. Reference Del Hoyo, Elliot and Christie2006). Spatial structuring might also be caused by the response to habitat features that are actively selected by the species, and are themselves spatially structured (Keitt et al. Reference Keitt, Bjørnstad, Dixon and Citron-Pousty2002). However, while the existence of significant differences in the physical structure of the habitat and floristics of different fragments is confirmed by the results of this and other studies (Wilder et al. Reference Wilder, Brooks and Lens2000, Chege and Bytebier Reference Chege and Bytebier2005), our data show that habitat taken alone has a scarce ability to predict the presence of the Taita Apalis. We therefore suggest that spatial structuring in the distribution of this species is largely a consequence of the strong differences in abundance that we found across different forest fragments. However, our data do not precisely show what causes these differences. Further research is needed to clarify these factors. Perhaps, they should be looked for at a scale (e.g. microhabitat) different to the one on which we focused, at a different season of the year or among factors not related to habitat such as nest predation, which is strong in the Taita and can vary considerably between different forest fragments (Spanhove et al. Reference Spanhove, Lehouck, Boets and Lens2009a,Reference Spanhove, Lehouck and Lensb). In particular, recent data show that nest predation is high in Chawia (Spanhove et al. Reference Spanhove, Lehouck and Lens2009b), suggesting that this factor should be considered as one potential cause of the low abundance of the apalis in this fragment.
Our work, and the results of previous studies (Brooks et al. Reference Brooks, Lens, Barnes, Barnes, Kageche Kihuria and Wilder1998a, Brooks et al. Reference Brooks, Lens, De Meyer, Waiyaki and Wilder1998b) show that the Taita Apalis only occurs in three of the 12 Taita Hills forest fragments (Ngangao, Chawia and Vuria, in total 257 ha). What is the reason for the absence of Taita Apalis from most of the Taita forest fragments? In the Taita Hills, bird persistence in forest fragments is largely determined by mobility and tolerance of habitat disturbance. These two factors alone explained 88% of the variation in forest occupancy in a group of eight bird species of the Taita Hills, where the most widespread species had either higher mobility or more ability to tolerate habitat disturbance (Lens et al. Reference Lens, Van Dongen, Norris, Githiru and Matthysen2002b). Despite intensive bird ringing efforts, dispersal across forest fragments has never been observed in Taita Apalis (Lens et al. Reference Lens, Van Dongen, Norris, Githiru and Matthysen2002b), suggesting that this species has a low mobility. On the other hand, a positive result emerging from our study is that Taita Apalis was frequent in the disturbed, scrub-like vegetation of Vuria. This suggests that the species is tolerant of the effects of woodcutting and disturbance by humans. Moreover, in an NMDS ordination, presence points of Taita Apalis were broadly scattered over the entire multivariate space, suggesting that the absence of the species from Yale, Fururu and Mbololo is not due to the absence of suitable habitat in these fragments. The absence of Taita Apalis from the majority of the Taita Hills forest fragments might therefore be a consequence of stochastic extinction and inability to recolonise these fragments due to low mobility. From a practical conservation point of view, reintroduction of Taita Apalis to the forest fragments from where it is currently absent should be possible at least with regards to the availability of suitable habitat in these fragments (even though other factors, such as predation should be taken into account). Human-aided translocation might compensate the low dispersal ability of this species.
In summary, we confirm that the conservation status of Taita Apalis is extremely precarious. We found the species in three forest fragments (Ngangao, Vuria, Chawia), whose total area is approximately 257 ha, but not in a fourth one (Fururu), where it had been observed in the past (Brooks et al. Reference Brooks, Lens, De Meyer, Waiyaki and Wilder1998b). These fragments are separated by a matrix of farmland and there is no evidence that the species can disperse between these fragments (Lens et al. Reference Lens, Van Dongen, Norris, Githiru and Matthysen2002b), making natural recolonisation of unoccupied sites unlikely. Moreover, Chawia supports such a low population (probably less than 50 individuals), that the survival of this subpopulation is unlikely in the long term in the absence of conservation actions. Taita Apalis thus qualifies for ’Critically Endangered’ under IUCN criterion B2a+b (Area of occupancy < 10 km2, severely fragmented and continuing decline in number of locations and subpopulations (IUCN 2001).
Acknowledgements
Funding for this research was provided by Wellcome Trust Grant No. 057622. Special thanks go to Silvester Karimi and Robert Kang’ara for their assistance gathering the data. We also thank Phil Atkinson, Steve Buckland, Thomas Brooks and Luc Lens for providing useful comments on an earlier draft of the paper, and the Kenya Forest Service and the Forest Guards of Taita District for their assistance.










