Introduction
The management of natural forests has shifted over time to maintain ecological integrity while meeting timber demands (Jõgiste et al. Reference Jõgiste, Korjus, Stanturf, Frelich, Baders, Donis and Jansons2017). One proposed strategy is that of near-to-natural forestry management which seeks to mimic the structure, composition, and processes of natural forests (Peterken Reference Peterken1999). To effectively employ the near-to-natural forestry strategy, a good understanding of forest biodiversity and ecosystem function is required. One facet of this is to understand how resource use may impact forest species’ habitat requirements at different stages in their life history, such as breeding, and how best to mitigate these impacts. Single-tree harvesting is a practice which aims to imitate natural, fine-scale disturbance regimes in forest systems and was developed to reduce the negative impacts of timber harvesting on forests (Franklin et al. Reference Franklin, Spies, Pelt, Carey, Thornburgh, Rae Berg and Lindenmayer2002). Whilst this practice has been shown to benefit biodiversity within managed forests (Tobler et al. Reference Tobler, Anleu, Carrillo-Percastegui, Santizo, Polisar, Hartley and Goldstein2018), there are some cases where the harvesting criteria used to select candidate trees resulted in the removal of a set of trees that formed an important part of a species’ – or group of species’ – habitat requirements (e.g. White and Jimenez Reference White and and Jimenez2017, Miranda et al. Reference Miranda, Peres, Marini and Downs2020). Thus, selective single-tree harvesting in some instances may result in habitat degradation.
In South Africa, selective harvesting of indigenous trees for timber takes place in two indigenous forest complexes, one being the Amathole forests in the Eastern Cape Province. The forests in this region have a complex history with regards to logging, having been extensively logged up until 1939 when indigenous tree harvesting was largely stopped (von Maltitz et al. Reference Von Maltitz, Mucina, Geldenhuys, Lawes, Eeley, Adie and Vink2003). However, between 1939 and when harvesting was formally resumed in 1975, opportunistic harvesting of wind-fallen, and dead or dying trees was permitted (King Reference King1941). Subsequently, harvest selection criteria have been refined, based on a mortality retrieval harvesting yield regulation system developed in the southern Cape forests (Seydack Reference Seydack1995) and implemented in the Amathole forests in the late 1990s (Vermuelen et al. 2000, Mpisekaya et al. 2008 unpublished report). This has resulted in the harvesting of wind-fallen, crownless, crown damaged, dead, and dying trees.
Although the mortality retrieval harvesting yield regulation system aims to minimise ecological impacts (Seydack Reference Seydack1995), the trees selected for harvesting under this system, i.e. those that are old, dying, or dead, form a vital resource as many cavity-nesting bird species rely heavily on such trees for nesting, roosting, and foraging and are thus vulnerable to single-tree selective harvesting practices that target these trees (Martin and Eadie Reference Martin and Eadie1999, Lindenmayer et al. Reference Lindenmayer, Laurance and Franklin2012). Economically, the size of these old trees gives them great value, and under the premise that trees approaching death or already dead no longer contribute to stand growth, their removal from the system is considered sustainable (Seydack Reference Seydack1995, Mpisekaya et al. 2008 unpublished report). However, the potential effect of single-tree harvesting based on mortality retrieval on nest availability for cavity-nesting birds remains unknown. Between 2009 and 2014, the number of threatened forest-dependent birds almost doubled from 10% to 19% in South Africa (Berliner Reference Berliner2009). Additionally, 50% of forest-dependent birds in South Africa experienced range declines between 1992 and 2015 (Cooper et al. Reference Cooper, Wannenburgh and Cherry2017). This can be attributed largely to extensive informal harvesting of forest products (Leaver and Cherry Reference Leaver and Cherry2020a), which affects avian community structure (Leaver et al. Reference Leaver, Mulvaney, Ehlers Smith, Ehlers Smith and Cherry2019b), and leads to harvest-mediated changes in habitat heterogeneity, negatively affecting forest specialist bird species (Leaver et al. Reference Leaver, Carstens and Cherry2019a). Cavity-nesting species are particularly vulnerable in South Africa, as shown by Cooper et al. (Reference Cooper, Norris and Cherry2020) using a trait-based assessment. Specifically, this study found that nesting traits were more important in determining risk than feeding traits for forest birds. Despite this, there have been few studies on cavity-nesting birds in the southern African context.
The Cape Parrot Poicephalus robustus is listed as “Vulnerable” by the IUCN and is South Africa’s only endemic parrot species (Birdlife International 2021). These parrots have a restricted range and breed only in the fragmented mosaic of Afromontane Mistbelt forests that spans from Hogsback in the Eastern Cape to the Magoebaskloof region in the Limpopo Province (Downs Reference Downs2005). While the distribution of the Cape Parrot was formerly more widespread, increased habitat loss, degradation, and fragmentation have resulted in major range contractions (Cooper et al. Reference Cooper, Wannenburgh and Cherry2017), although its population size has remained stable in this century (Downs et al. Reference Downs, Pfeiffer and Hart2014). The largest sub-population of breeding Cape Parrots, consisting of ~900 individuals is found in the Amathole Mistbelt forests of the Eastern Cape, which is considered the breeding stronghold of this species. Contemporary selection criteria for timber harvesting in the Amathole forests cause an overlap in traits between candidate trees for harvesting and preferred nesting sites for the Cape Parrot, resulting in a potential harvest-mediated decline in Cape Parrot nest tree availability by close to a third (Leaver et al. Reference Leaver, Carstens, Wimberger, Carstens and Cherry2023). Specifically, the Cape Parrot relies on two yellowwood species, Afrocarpus falcatus and Podocarpus latifolius, for nesting (Wirminghaus et al. Reference Wirminghaus, Downs, Perrin and Symes2001, Carstens et al. Reference Carstens, Carstens and Wimberger2022), which are also the target species of formal harvesting activities in the Amatole forests (Vermeulen et al. Reference Vermeulen, Maseti and Kameni2000, Leaver et al. Reference Leaver, Carstens, Wimberger, Carstens and Cherry2023). Cape Parrots do not excavate their own nesting holes, but rather modify natural cavities which develop in tree trunks when branches fall off, requiring older trees in the early stages of decay to create suitable nests (Wirminghaus et al. Reference Wirminghaus, Downs, Perrin and Symes2001, Leaver et al. Reference Leaver, Carstens, Wimberger, Carstens and Cherry2023). The three primary cavity-excavating bird species found in the Amathole forests, which create holes that may provide additional tree holes to be modified and used by parrots, are the Knysna Woodpecker Campethera notata, Olive Woodpecker Dendropicos griseocephalus, and Red-fronted Tinkerbird Pogoniulus pusillus. The Olive Woodpecker and Red-fronted Tinkerbird are classified as “Least Concern”, whilst the Knysna Woodpecker is listed as “Near Threatened” (Birdlife International 2021). All three species rely on dead or decaying wood for excavating cavities (Tarboton Reference Tarboton2011), although the extent to which Cape Parrots use tree cavities made by these primary excavating species for breeding is largely unknown.
Acoustic recording units (ARUs) are becoming increasingly popular bio-acoustical monitoring tools to record the presence or absence of avian species in specific regions (e.g. Drake et al. Reference Drake, de Zwaan, Altamirano, Wilson, Hick, Bravo and Ibarra2021). ARUs can additionally provide insight as to the breeding status for species having documented breeding calls, as is the case for the Cape Parrot (Wirminghaus et al. Reference Wirminghaus, Downs, Symes, Dempster and Perrin2000). This study aimed to use ARUs to investigate the presence or absence of the Cape Parrot and three primary excavating species in 16 forests with differing levels of logging in the Amathole region of the Eastern Cape. We predicted that the extent of harvesting in a forest would negatively affect the presence of these four cavity-nesting bird species. Additionally, we investigated whether logging intensity of yellowwoods affected the breeding status of Cape Parrots in the same forests. Again, we predicted that logging intensity would negatively affect the breeding status of Cape Parrots. Third, we aimed to identify forests where parrots breed, as these are of particular importance for their conservation.
Methods
Study area and tree harvesting
The study was conducted in the Amathole region of the Eastern Cape, South Africa (Figure 1). Within this region the forests are classified as Amathole Mistbelt, with the forests included in this study comprising the Amathole Escarpment subtype which occur along mountain slopes between 500 m and 1,600 m altitude (von Maltitz et al. Reference Von Maltitz, Mucina, Geldenhuys, Lawes, Eeley, Adie and Vink2003). This is the second largest indigenous forest complex in South Africa, covering an estimated 35,000 ha (Von Maltitz et al. Reference Von Maltitz, Mucina, Geldenhuys, Lawes, Eeley, Adie and Vink2003), and is managed by the Department of Environment, Forestry and Fisheries (DFFE). The region experiences a temperate climate with an annual average rainfall of between 800 mm and 1,800 mm, falling mostly during the summer (October–February), with some rain falling in winter with an average of 400 mm per year (von Maltitz et al. Reference Von Maltitz, Mucina, Geldenhuys, Lawes, Eeley, Adie and Vink2003). Temperatures range between a minimum of 1˚C to a maximum of 29˚C, although the mean temperature is relatively mild, falling between 14˚C and 19˚C. Contemporary records of all trees formally logged were obtained from the DFFE for 16 different forests across the complex (Figure 1). The records covered 18 years during 1999–2017, as no logging has taken place since then owing to a lack of demand. From these data the number of trees (stems) harvested per square kilometre in the different forests could be assessed. Google Earth was used to map the area of each forest.

Figure 1. Map showing the location of forests within the Amatholes in Eastern Cape Province of South Africa. Numbers refer to forests listed in Table 1.
Data collection
Audio data were collected in each forest in two seasons, with the first lasting from July 2019 to March 2020 and the second from September 2020 to March 2021 (the second recording period commenced later on account of the SARS-COV-19 pandemic). Forests were randomly selected across the Amathole range. Wildlife Acoustics Song Meter SM4 ARUs were used to record audio data at two sites within each forest: one at the centre of the forest and the other at the edge. Sites were selected based on ease of access along existing trails and tracks, and spaced more than 800 m apart across as wide an area of the forest as possible. The ARUs recorded audio at a 44,100 Hz sampling rate in one-hour segments from half an hour before sunrise until half an hour after sunset. ARUs were deployed for seven days per season at each site, and attached to small to medium-sized trees with nylon straps and secured with cable locks and small padlocks.
Analyses
Audio analysis
Analysis of audio data was performed in Kaleidoscope (Wildlife Acoustics). Existing audio files of each of the species’ calls were obtained from xeno-canto.org, which were used to build a simple classifier. A simple classifier was used to sort through the inputted audio files and sort and cluster similar vocalisations. These clustered and sorted vocalisations were manually checked to see if they contained the focal species’ vocalisation. Data for analyses were screened first from the acoustic monitor closest to the centre of each forest patch, and, if the species was not detected there, then analyses were repeated using data from the monitor closest to the forest edge. The simple classifiers for the focal species were based on the characteristics of calls recorded in the Amathole region of the Eastern Cape. For Cape Parrots, the classifier parameters were set for a frequency range between 3,500 Hz and 6,000 Hz, a length between 0.1 second and 4.5 seconds, and an inter-syllable gap of 0.35 seconds for general contact calls, and 2,500–4,500 Hz, a length between 2 seconds and 8 seconds, and an inter-syllable gap of 0.5 seconds for breeding-related calls, reflecting call parameters of Cape Parrots in the Amatholes (J. C. Carstens, pers. comm.). For the other focal species, calls were also sourced from xeno-canto.org. For Knysna Woodpecker the frequency range was set between 3,200 Hz and 4,600 Hz and a length of 0.3–0.6 seconds. As this bird’s vocalisation consists of a single-syllable call, an inter-syllable gap was not included. For Olive Woodpecker the frequency range was set between 2,250 Hz and 4,250 Hz, a time length of 3.75–4.25 seconds, and an inter-syllable gap of 2.5–5.25 seconds. For Red-fronted Tinkerbird the frequency range was 1,000–1,500 Hz and an inter-syllable gap of 0.1 seconds. As the calls of this species last for more than 30 seconds, the time length parameter was not included.
Statistical analysis
All statistical analyses were conducted in R-Studio (Version 1.4.1103) (R Core Team 2020). A logistic regression (GLMM) was used to investigate the effect of yellowwood logging on parrot breeding status. The presence or absence of a breeding call was the response variable, and yellowwood trees harvested per unit area was the predictor variable, as these two are the only indigenous tree species in which breeding has been recorded in the Eastern Cape (Carstens et al. Reference Carstens, Carstens and Wimberger2022); forest was included as a random factor.
Results
Results from the acoustic data analysis showed that the Cape Parrot was present in 15 out of the 16 forests sampled, with Lushington Crown being the only forest where the species was absent (Table 2). However, Cape Parrot breeding calls were recorded in only 7 of the 16 forests, of which five were logged and two not (Table 1). Of the primary-excavating species, Olive Woodpecker and Red-fronted Tinkerbird were each absent in a single forest, i.e. Kologha and Mount Thomas, respectively, and Knysna Woodpecker was detected in all of the forests (Table 2). Neither of the two forests from which at least one excavating species was absent harboured breeding parrots (Table 1).
Table 1. Cape Parrot breeding calls as determined by acoustic monitoring and logging history 1997–2021 in the Amathole forests of Eastern Cape Province of South Africa.
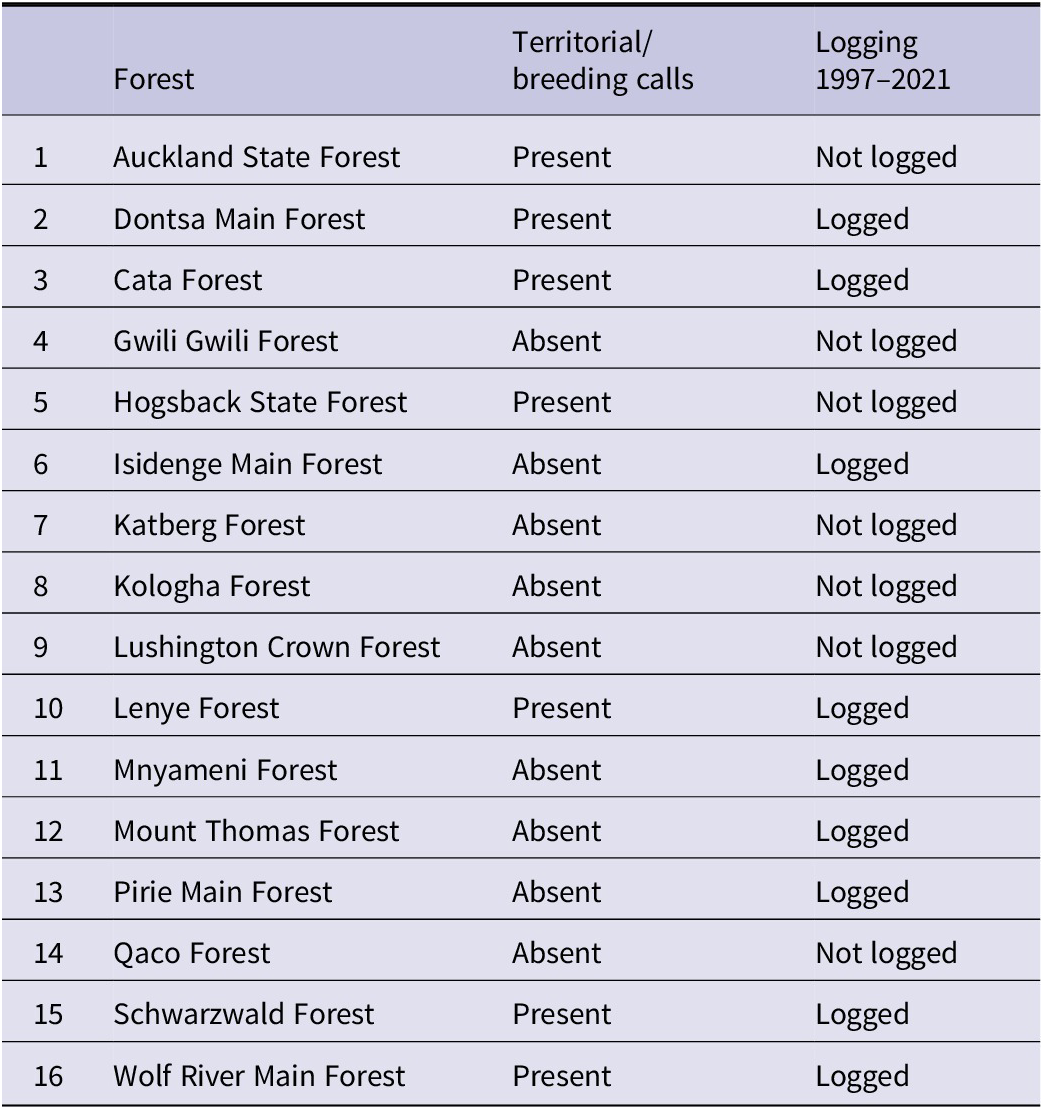
Table 2. Presence of cavity-nesting bird species, and authorised logging in the Amathole forests 1997–2021. Tree species which comprised 5% or more of logged species are listed.
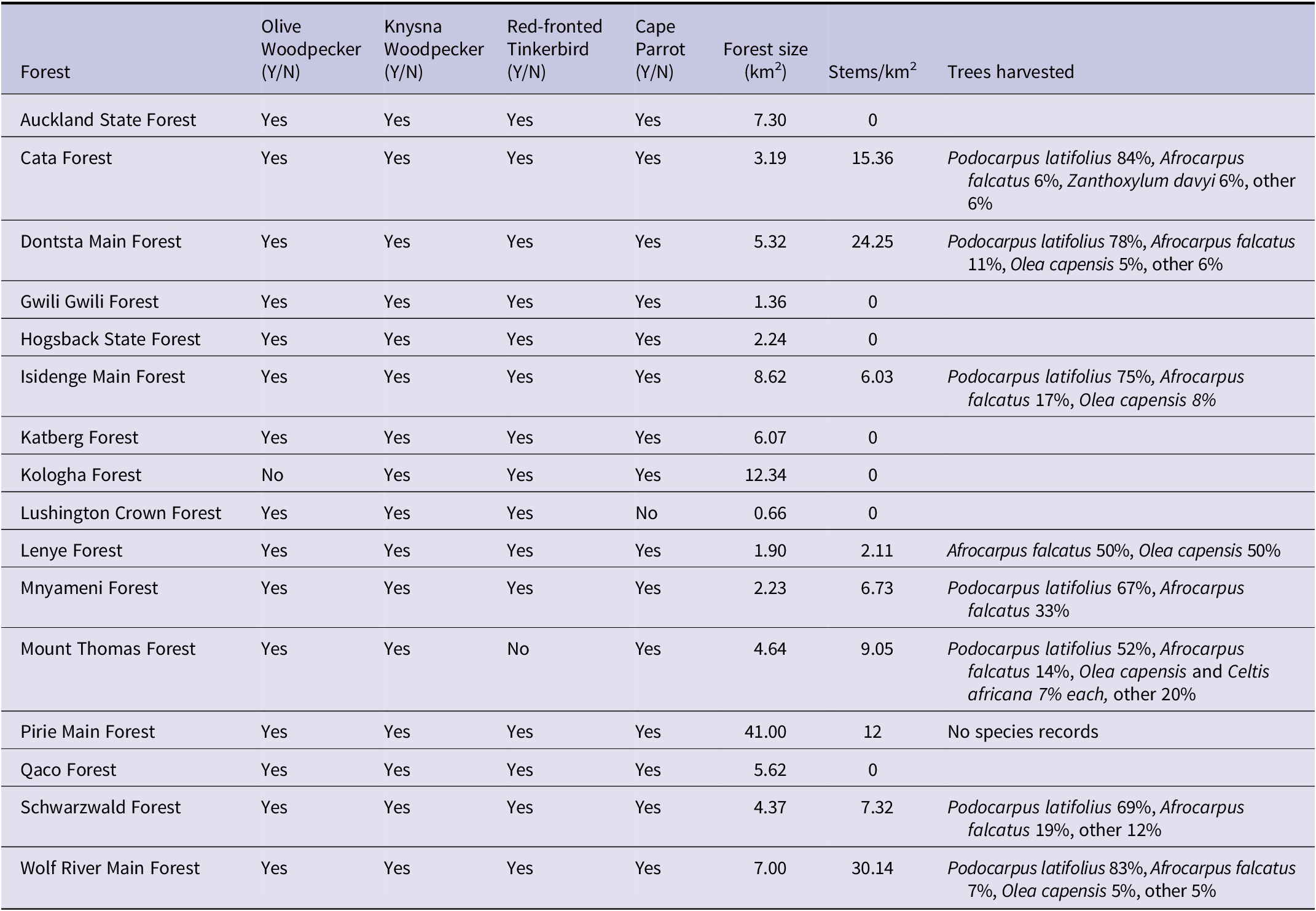
Selective logging occurred in 9 of the 16 forests, with 10 different tree species harvested (Table 2). Wolf River Main Forest had the highest density of trees harvested per unit area at an average of 30 stems/km2. The mean number of stems harvested from forests where harvesting had taken place was 13.32 stems/km2 ± 9.02 (range of 2.11–30.14 stems/km2). P. latifolius was harvested the most in all logged forests except for Lenye forest, where A. falcatus and Olea capensis were harvested in equal numbers (Table 2). A. falcatus was the second most harvested tree in seven of the nine forests (Table 2). Mount Thomas forest, where the Red-fronted Tinkerbird was undetected, had a moderate 9 stems/km2 harvested, but did have the largest variety of trees (nine different species) harvested (Table 2). Kologha forest, where the Olive Woodpecker was undetected, has experienced no logging in the past 25 years.
The presence of breeding parrots did not appear to be dependent on the intensity of logging of the Cape Parrot’s two preferred nesting trees, A. falcatus and P. latifolius (Table 3). This applied both when all 16 forests, and only logged forests (n = 9), were considered. There was, however, a non-significant trend for parrot breeding to be positively associated with the intensity of logging of yellowwood trees (Table 3).
Table 3. Response of Cape Parrot breeding to yellowwood harvesting intensity in all Amathole forests (n = 16) compared with only logged forests (n = 9), with forest included as a random effect.

Discussion
Contrary to our predictions, the results of the study suggested that selective logging over the past 25 years in the Amatholes does not have a significant effect on the presence or absence of the four focal cavity-nesting bird species; nor does it appear to have deterred breeding activity by Cape Parrots. Whilst a variety of different tree species were harvested, the majority of harvested trees were two species of yellowwoods, P. latifolius and A. falcatus (Table 2). The removal of mostly dead or dying, large, old yellowwoods, as per the mortality retrieval harvest regulation system used in the region, would reduce the abundance of suitable trees for the three primary cavity-nesting species, which rely on dead or decaying wood in which to excavate their cavities (Tarboton Reference Tarboton2011). In some forests, selective logging over the past 18 years has likely reduced the number of suitable trees available to excavate, although the effect is only occasionally large enough to cause an absence as there are enough other species of trees suitable for nesting, and only the Cape Parrot (Leaver et al. Reference Leaver, Carstens, Wimberger, Carstens and Cherry2023) and not the three primary excavators (Tarboton Reference Tarboton2011), show a strong preference for yellowwoods as nest sites. Historically, P. latifolius has been the preferred species of yellowwood to harvest rather than A. falcatus, not only because it was more abundant, but because its timber is of a higher quality (T. Stehle pers. comm.). While this still holds true, over the past 25 years, A. falcatus has become more frequently logged than previously. Cape Parrots nest predominantly in A. falcatus and while this species is relatively scarce compared with P. latifolius, a larger proportion of available stems of this species are of significant size (i.e. >100 cm DBH) compared with P. latifolius (Leaver et al. Reference Leaver, Carstens, Wimberger, Carstens and Cherry2023). Additionally, the softer timber of A. falcatus may make it more susceptible to natural cavity formation (Leaver et al. Reference Leaver, Carstens, Wimberger, Carstens and Cherry2023), but again harvesting levels have mostly not yet been high enough to prevent breeding within logged forests.
Many bird species that nest in cavities can be limited by a shortage of suitable nest sites, which can result in birds being excluded from an area (Newton Reference Newton1994). Although a species may have been detected in a forest where high levels of logging had taken place, the abundance of the species may be significantly lower than in an unlogged forest, yet in our study this would remain undetected. In a study on Greater Spotted Woodpeckers in Morocco, for example, more detections of the species were recorded in stands with a higher density of dead trees (Segura Reference Segura2017). One of the shortcomings of our study is that the presence/absence data prided by ARUs does not allow for species abundance to be measured.
Another shortcoming is that the logging data provided for this study were available only for the past 25 years. Logging has been taking place in the Amathole from as early as the late nineteenth century (Seydack and Vermuelen Reference Seydack and Vermeulen2004), but there are no available records from the first century of logging. This may be important within the Amathole region, as the current structure and composition of mistbelt forests in South Africa has been affected by extensive logging in the nineteenth and twentieth centuries (Lawes et al. Reference Lawes, Griffiths and Boudreau2007, Adie et al. Reference Adie, Rushworth and Lawes2013). This harvesting resulted in the removal of most trees above the minimum harvestable diameter at the time, reducing the number of larger trees currently approaching senility, particularly in the case of yellowwoods (Seydack and Vermuelen Reference Seydack and Vermeulen2004). In some Amathole forests, the mean annual DBH of yellowwoods harvested has decreased over the past 25 years, suggesting a decline in larger trees (Leaver et al. Reference Leaver, Carstens, Wimberger, Carstens and Cherry2023). A decline in the number of large dead or dying, standing trees (snags) in a forest is of concern as larger snags remain standing for longer, thereby allowing a variety of cavities to form naturally or be excavated over time (Newton Reference Newton1994). Moreover, snags play an important role in the foraging of many bird species (Drapeau et al. Reference Drapeau, Nappi, Imbeau and Saint-Germain2009). Consistent removal of large old trees has thus changed both the structure and functioning of the Amathole forests, some more than others.
The two absences of primary cavity-nesting birds were recorded in Kologha and Mount Thomas forests, near the town of Stutterheim, where parrot breeding was also not recorded. These absences may reflect high levels of historical logging in these forests given their proximity to a large settlement, or due to other contemporary anthropogenic disturbances (both forests are fragmented in a matrix of exotic plantations). Nyirenda et al. (Reference Nyirenda, Chewe, Chisha-Kasumu and Lindsey2016) found that miombo woodland with high levels of current anthropogenic disturbance had significantly lower numbers of cavity-nesting birds and three times fewer cavities than undisturbed woodland. The nine forests in which no parrot breeding was recorded include Pirie, which contained one of the first operational sawmills in South Africa and which historically was heavily logged (Wells Reference Wells1973, McCracken Reference McCracken, Lawes, Eeley, Shackleton and Geach2004). Pirie, as well as Gwili Gwili and Isidenge, are situated closest to King Williams Town, a well-known wagon-making town in the eighteenth and nineteenth centuries, which was also the first town in the country to obtain a fully functional sleeper creosoting factory (Wells Reference Wells1973). In 1892 a railway line, still in operation, was established north of Queenstown in the Eastern Cape. Katberg and Qaco forests, in which no Cape Parrot breeding calls were detected, are the closest to this railway line, which facilitated the removal of extensive amounts of timber from the region to other parts of the country (McCracken Reference McCracken, Lawes, Eeley, Shackleton and Geach2004). There may of course also be non-anthropogenic reasons why particular forests do not harbour suitably large yellowwood trees in which parrots can breed. Direct counting of yellowwood trees of suitable size, either from the ground (Leaver et al. Reference Leaver, Carstens, Wimberger, Carstens and Cherry2023) or using light detection and ranging (LIDAR), would provide a more accurate measure of suitable breeding sites in each forest.
The Amathole forests may be experiencing an additional anthropogenic disturbance in the form of fuelwood harvesting, which could alter the availability of dead wood. The Eastern Cape is one of the poorest provinces in South Africa, thus many people rely on fuelwood as a cost-effective and reliable energy source (Kaoma and Shackleton Reference Kaoma and Shackleton2015). Guild and Shackleton (Reference Guild and Shackleton2018) found that 80% of urban centres sampled in Limpopo and the Eastern Cape had one or more informal fuelwood markets, showing both the continued importance of wood as an energy source and as a traded commodity. Whereas the presence of informal harvesting of bark, timber, and poles is easily observable, and the extent of harvesting thus quantifiable (Leaver et al. Reference Leaver, Carstens and Cherry2019a), informal harvesting of fuelwood is harder to quantify as one must rely on estimates provided by harvesters. Social surveys have found that fuelwood harvesting takes place in Pirie Forest, which is near several villages, and that yellowwoods, particularly A. falcatus, are used for fuelwood as well as for building material (Gugushe et al. Reference Gugushe, Grundy, Theron and Chirwa2008, Opperman et al. Reference Opperman, Cherry and Makunga2018). However, informal harvesting within the Amathole forests appears to be relatively limited (Leaver and Cherry Reference Leaver and Cherry2020a), and is thus unlikely to significantly impact forest structure (Leaver and Cherry Reference Leaver and Cherry2020b). Moreover, while the demand for fuelwood persists, supply is increasingly being met through non-forest sources, such as managed (i.e. woodlots) or unmanaged stands of exotic species (e.g. Black Wattle, Gum, and Australian Blackwood), as well as the increasing abundance of native woody encroacher species such as Vachellia karroo.
Cape Parrots appear not to be heavily dependent on primary excavators for nesting sites as most (95%) of nesting sites in the Amatholes are natural cavities arising from rotting of the tree core, either on the main stem or after a side branch has broken off (J. C. Carstens pers. comm.). Nonetheless, this study highlights the necessity for further research on the relationships between the Cape Parrot and primary excavating species that make holes which it could modify as nests, particularly in the context of cavity-nesting species being at risk in South African forests on account of a paucity of nesting sites (Cooper et al. Reference Cooper, Norris and Cherry2020). We suggest that in future ARUs could be used in a more comprehensive investigation into the impact of harvesting on Cape Parrot and primary cavity excavators’ breeding activity, by determining whether gradients of harvesting intensity within harvested forests, measured either from the ground or using LiDAR, relate to breeding activity.
Logging activities over the past quarter-century did not appear to have had a significant effect on the presence of three primary excavating species, nor on Cape Parrot presence or breeding activity. However, our study illustrates the utility of acoustic monitoring units in determining both presence and breeding in cavity-nesting species, where finding nests is often both difficult and labour-intensive. Our study highlights absences of primary cavity-nesting species in Mount Thomas and Kologha (where parrots are present but were not recorded breeding), suggesting a lack of suitable nesting or foraging sites for these species in this area. Furthermore, it shows that Cape Parrot breeding occurs in five logged forests, where there is an overlap in terms of trees which meet current harvesting criteria and suitable nesting sites for parrots. As demand for indigenous timber is low, with no formal harvesting having taken place in the past four years, we recommend that logging in these five forests in which parrots are currently breeding be limited to tree falls, with no standing dead, dying, or damaged trees harvested, on account of this overlap (Leaver et al. Reference Leaver, Carstens, Wimberger, Carstens and Cherry2023).
Acknowledgements
We thank Wildlife Acoustics for the sponsorship of the acoustic monitors used in the project, and the Wild Bird Foundation and Wild Bird Trust for staff and operational funding. Theo Stehle is thanked for information regarding the history of logging within the Amathole forests.






