Introduction
Septic arthritis (SA) is serious. Mortality is greater than 10%, and close to half of the patients have sequelae related to irreversible damage to cartilage and joint destruction.Reference Dubost, Soubrier and Sauvezie 1 The prognosis is worsened by any therapeutic delay, so early diagnosis is essential.
The diagnosis of SA is confirmed by the detection of a microorganism in the joint. However, direct Gram stain is positive in only 25% to 50% of cases, and culture requires a delay.Reference Margaretten, Kohlwes, Moore and Bent 2 Bacteriological findings remain negative in approximately 20% of SA cases in the literature, thus the diagnosis is presumptive.Reference Eberst-Ledoux, Tournadre and Mathieu 3 Numerous pathologies, notably crystal-induced and rheumatic diseases, may present in a fashion identical to SA.Reference Eberst-Ledoux, Tournadre and Mathieu 3 In a patient with suspected SA, it is often difficult to evaluate the probability of SA in order to decide on hospitalization or initiation of antibiotic therapy. The sensitivity of clinical signs and laboratory tests is published in a single case series of 74 patients, but large disparities exist due to bias in recruitment and the choice of inclusion criteria. There is no study available on the specificity of clinical signs, and the data on the specificity of laboratory tests are very limited.Reference Margaretten, Kohlwes, Moore and Bent 2 , Reference Carpenter, Schuur, Everett and Pines 4
This is a prospective study aimed to establish the predictive value of clinical signs and laboratory tests for the diagnosis of SA in patients referred with a suspicion of SA.
Method
This is a prospective observational cohort study carried out in the emergency and rheumatology departments of the University Hospital, Clermont-Ferrand (primary and secondary referral centre located in a rural region where acquired immune deficiency syndrome [AIDS] and drug abuse are rare). All consecutive patients suspected of having an SA and examined by a rheumatologist over a period of 18 months (between July 2010 and January 2012) were enrolled. There is no validated definition for suspected SA. In order to be as pragmatic as possible and reflect general routine practice, we chose to include all patients for whom the rheumatologist had been contacted for a suspicion of SA independent of the duration, clinical presentation, site, or number of joints involved. Excluded from this study were patients under the age of 18 or those with a joint prosthesis or joint trauma.
For every patient included, informed consent was obtained, and standardized clinical data were recorded. Data included demographics (age, sex), number of joints affected, duration (in weeks), and timing of the onset of symptoms (acute if less than 24 hours or gradual if more than 24 hours), fever (>38°C [100°F]), chills, local inflammatory symptoms, visual analogue scale (VAS) for pain at rest and upon movement, localized adenopathy, the existence of an entry site for infection, history of rheumatic disease (rheumatoid arthritis, ankylosing spondyloarthritis, polymyalgia rheumatic, or other) or crystal-induced arthritis (gout, chondrocalcinosis), risk factors for SA (diabetes mellitus, cancer, immunosuppression, alcoholism, renal insufficiency, chronic corticosteroid therapy, or other), treatments received prior to evaluation (nonsteroidal anti-inflammatory agents, colchicine, or antibiotics), as well as the presence of an extra-articular site of infection (endocarditis, spondylodiscitis).
The results of the usual laboratory tests were also recorded (white blood cells [WBC]/mm3, polymorphonuclear cells [PMN]/mm3, erythrocyte sedimentation rate [ESR] in millimeters at the first hour, C-reactive protein [CRP] in milligrams per litre [mg/L], uric acid in micromoles per litre [µmol/L]). The radiological findings suggestive of SA (i.e., reduced joint space width, subchondral demineralization, erosion, or joint destruction) as well as the presence of chondrocalcinosis or a pre-existing arthropathy were recorded. Joint ultrasound results were noted if performed. If a joint aspiration were performed, the gross appearance of synovial fluid would be classified by the rheumatologist as clear, turbid, purulent, or hemorrhagic. The synovial fluid was analysed by the bacteriology laboratory for cytology, presence of microcrystals, Gram stain, and culture, while a portion of the fluid was systematically injected into a blood culture tube at the patient’s bedside. After the previous examinations and before receiving the analysis of the synovial fluid, the rheumatologist evaluated the probability of SA using a VAS and indicating and documenting the most probable diagnosis. The final diagnosis was made a posteriori using the results of all of the investigations and the course of the disease. In the end, patients were classified into two groups: 1) an SA group, if it was certain (i.e., identification of a microorganism in the joint fluid or via blood culture) or possible (i.e., prescription of antibiotic therapy for at least 3 weeks); or 2) a non-SA group, in cases of crystal-induced arthritis, rheumatic disease, or other defined diagnosis.
This study was conducted under the authorization of the local ethics committee (CPP Sud-Est I, 28/5/2010) and in line with regulatory authorities (12/09/2009).
Statistical analysis
The population was described by the number/percentage for categorical variables and by the mean (+/− standard deviation) or median (interquartile interval) for the continuous variables. The normality of the quantitative variables was verified by the Shapiro-Wilk test, and a log transformation was performed when the test was significant. Quantitative data were compared between SA and non-SA groups using the Student’s t-test or the Mann-Whitney test when the Student’s t-test conditions were not verified. Categorical data were compared by the chi-squared test or the Fisher’s exact test when appropriate. For the 2×2 contingency tables, the data relating to sensitivity, specificity, negative likelihood ratios (LR−) and positive likelihood ratios (LR+) of all signs and symptoms used to differentiate SA from other causes of similar joint damage were calculated and presented with the 95% confidence interval (CI) as well as odds ratios (OR). Logistic regression models (with dependent variable SA or non-SA) established the predictive value of clinical signs and laboratory tests for the diagnosis of SA, employing a backward and forward stepwise regression on the factors considered significant in the univariate analysis (entry in the model for p<0.2Reference Lee and Koval 5 , Reference Shtatland, Cain and Barton 6 ) and considering clinical relevance parameters,Reference Hosmer and Lemeshow 7 , Reference Steyerberg, Eijkemans, Harrell and Habbema 8 such as antecedents of rheumatic disease and risk factors (adjustment factors). Results were expressed as OR and 95% CI. Following these multivariate analyses, a receiver operating characteristic curve was plotted for each proposed model, and their areas under the curve (AUC) were compared.Reference DeLong, DeLong and Clarke-Pearson 9 Tests were two-sided, with a type I error set at α=0.05. All analyses were performed using STATA (version 11, StataCorp, College Station, Texas).
Results
In total, 105 patients with suspected SA were included and of these 29 (27.6%) were confirmed SA and 9 (8.6%) were SA-possible. The most frequently affected joints after the knee (n=63, 60%) were hip (n=15, 14.3%), wrist (n=11, 9.5%), ankle (n=9, 8.5%), shoulder (n=7, 6.6%), metacarpophalangeal joint (n=5, 4.8%), elbow (n=5, 4.8%), and metatarsophalangeal joint (n=4, 3.8%), with 15 patients (14.3%) presenting with involvement of more than one joint.
For the 29 patients for whom SA was confirmed, the most frequently identified microorganisms were methicillin-sensitive Staphylococcus aureus (n=17, 58.6%), Streptococcus (n=3, 10.3%), and methicillin-resistant S. aureus (n=2, 6.7%). Other germs were identified (Enterobacter aerogenes, Klebsiella pneumoniae, Escherichia coli, Campylobacter spp, Salmonella typhimurium, Neisseria gonorrhoeae, and coagulase-negative Staphylococcus).
For all of the nine SA-possible patients, the course of the disease was compatible with the diagnosis of SA with improvement after antibiotics and no other diagnosis considered a posteriori in the following 6 months.
For the 67 patients without SA (non-SA group), the final diagnosis was, in most cases, crystal-induced arthritis (n=35, 33.3%) (15 patients with gout and 20 with chondrocalcinosis), followed by rheumatic disease (n=11, 10.5%) (8 patients with rheumatoid arthritis, 2 with spondylarthropathy, and 1 with polymyalgia rheumatica). In addition, six patients (5.7%) had undifferentiated arthritis, six (5.7%) had osteoarthritis, four (3.8%) had hemarthrosis, and five (4.8%) had another diagnosis (one hematoma of the psoas, enthesopathy of the right rectus femoris, one hydroxyapatite deposition disease, allergic arthritis following viscosupplementation, and popliteal cyst rupture). The clinical characteristics are shown in Table 1.
Table 1 Patients clinical characteristics

Expressed in number (%) or mean±SD.
*: p<0.05 in univariate analysis.
Patients with SA were younger than those from the non-SA group (58.6 v. 60.5 years, p=0.03), duration of symptoms was shorter in the non-SA group (1.8 v. 2.6 weeks, p=0.04), and acute onset (<24 hours) was more frequent (72.3% v. 46%, p=0.008). When the involved joint was the knee, it was most often non-SA (68.7% v. 42.1%, p=0.008). Involvement of more than one joint was similar in both groups (p=0.41).
The SA group had chills more frequently (39.5% v. 17.9%, p=0.01) but no fever, and local redness (52.6% v. 28.4%, p=0.01). There was no significant difference for local swelling, increased cutaneous heat, tenderness, presence of adenopathy or an extra-articular localization, history of rheumatic disease, risk factors for SA, as well as previous treatments. The presence of an entry site for infection was more common in the SA group (71.1% v. 46.3%, p=0.01). In the non-SA group, history of crystal-induced disease was more frequent (28.4% v. 5.3%, p=0.004).
Serum laboratory values
No difference was found for the WBC count, PMNs, and uric acid (329±27 mg/L in the SA group v. 346±20 mg/L in the non-SA group). The ESR and CRP levels were higher in the SA group (respectively as mean (±SD), 76.1±39.8 v. 45.7±33.8 mm/h [p=0.002] and 135.1± 16.7 v. 95.1±13 mg/L [p=0.015]). An ESR>50 mm/h (p=0.005) and CRP>100 mg/L (p=0.019) were more commonly observed in SA.
Radiography and joint ultrasound
Radiological findings suggestive of SA were found more often in patients with SA (29.7 v. 5.1%, p=0.001), and there was no difference in the radiological findings of chondrocalcinosis. A joint ultrasound was performed on 48 patients (45.7%), and there was no difference in the presence of an effusion between the two groups.
Synovial laboratory test values
Joint aspiration was performed in 90% of the cases (n=94). The appearance of the fluid was more commonly purulent (74% v. 26%, p<0.001) in cases of SA, and clear (100% v. 0%, p=0.007) in cases of non-SA. The SA group had a higher mean (95% CI) synovial WBC count (54,900 [20,000–112,000]/mm3 v. 15,000 [5,000–36,200], p<0.001) as well as a higher level of PMNs (85% v. 72%, p=0.001). There was no difference regarding the presence of microcrystals. The direct Gram stain was positive in 40% (n=14) of SA cases.
Opinion of the clinician
Estimation of the probability of SA before receiving the results of the joint fluid analysis was higher in the SA group (69.4% v. 31.4%, p<0.001). The clinician’s opinion was incorrect for 10 patients: 5 with a final SA diagnosis and the other 5 with a final non-SA diagnosis.
The probability of SA estimated by the clinician was correlated to symptom duration (p=0.008), fever (p=0.002), chills (p=0.006), local redness (p=0.004), existence of an entry site for infection (p=0.003), history of crystal-induced arthritis (p=0.03), serum WBC count (p=0.025), ESR (p=0.003), CRP level (p=0.004), radiological findings of SA (p=0.02), gross appearance of the synovial fluid (p<0.001), and synovial WBC count (p=0.02).
The sensitivity, specificity, likelihood ratios, and odds ratios of the clinical signs, laboratory tests, radiological, and ultrasound findings are shown in Table 2.
Table 2 Sensitivity and specificity, positive and negative likelihood ratios, and odds ratios of signs for diagnosing septic arthritis

ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; PMN: Polymorphonuclear cells; LR: likelihood ratio; OR: odds ratio; CI: confidence interval
Multivariate analysis
In the multivariate analysis, chills (OR=4.7, 95% CI 1.3–17.1), history of crystal-induced arthritis (OR=0.09, 95% CI 0.01–0.9), purulent appearance of the synovial fluid (OR=8.4, 95% CI 2.4–28.5), synovial WBC count>50,000/mm3 (OR=6.8, 95% CI 1.3–36), and suggestive radiological signs (OR=7.1, 95% CI 13–37.9) were significantly associated with SA.
Two logistic regression models, one with and one without radiological findings, were selected (Figure 1). Model 1 includes the parameters: chills, history of crystal-induced arthritis, radiological findings of SA, and purulent appearance of the synovial fluid; the AUC was 0.84. This model may correspond to the usual practice in an emergency, when the clinician has the clinical and radiological parameters and the appearance of the synovial fluid but not the results of its analysis. Model 2 includes the parameters: chills, history of crystal-induced arthritis, synovial WBC>50,000/mm3, existence of an entry site for infection, and risk factors for SA; the AUC was 0.87.
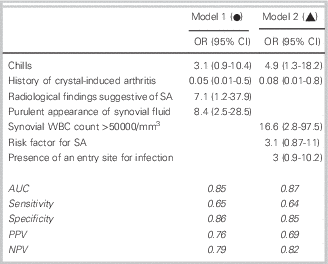

Figure 1 Logistic regression models and ROC curves for the diagnosis of septic arthritis. OR: odds ratios; CI: confidence interval; AUC: area under the curve; NPV: negative predictive value; PPV: positive predictive value.
The univariable and multivariable analysis was repeated, excluding all of the “possible” SA patients (n=9) without any changes in the outcomes.
Discussion
In our cohort of patients with suspected SA, 36% were confirmed or probable SA. The presence of chills, a history of crystal-induced arthritis, radiological findings suggestive of SA, as well as the gross appearance of the synovial fluid and its cellularity were the parameters that best differentiated SA from non-SA cases.
While waiting for the bacteriological results, the diagnosis of SA is based on a review of risk factors, as well as clinical signs and laboratory tests. Age>80 years, diabetes, and rheumatoid arthritis, which are risk factors for SA, have a comparable frequency in SA and non-SA groups.Reference Kaandorp, Van Schaardenburg and Krijnen 10 On the contrary, a history of crystal-induced arthritis (gout or chondrocalcinosis) was more frequent in the non-SA group (28% v. 5%), and its presence decreased the probability of SA (LR+0.58). In our study, chills appeared to be more frequent, present in 40% of SA cases, and an independent factor in multivariate analysis; however, the LR+ was only 2.2 and its sensitivity was weak, notably 19% based on a prior study.Reference Margaretten, Kohlwes, Moore and Bent 2 The serum WBC count did not differ between the SA and non-SA groups. An ESR>50 mm at 1 hour and a CRP>100 mg/L were weakly associated with SA. The specificity was mediocre in the few studies that evaluated this parameter,Reference Li, Cassidy and Chang 11 , Reference Ernst, Weiss, Tracy and Weiss 12 and a recent review of the literature concluded that, regardless of the threshold used, ESR and CRP levels did not significantly increase the posttest probability of SA.Reference Margaretten, Kohlwes, Moore and Bent 2
Thirty percent of patients already had radiological findings suggestive of SA at diagnosis. Although they were associated with a major diagnostic delay, radiological findings suggestive of SA remain useful (LR+5.8). In prior cases series, these signs were present in approximately half of the SA casesReference Dubost, Soubrier and Sauvezie 1 ; however, the specificity was not reported. The definition of the radiological findings (i.e., reduced joint space width, bone demineralization, erosion, and destruction) poses a problem. The frequency of radiological findings of chondrocalcinosis was not significantly different between the two groups.
The gross appearance of synovial fluid as evaluated by the clinician was a valid parameter in excluding SA when the fluid was clear (sensitivity null) or to consider it when it was purulent (LR+4.7). In any case, its interpretation is often subjective and difficult to standardize. The cellularity of synovial fluid was considered to be the best non-bacteriological test for the diagnosis of SA. In a review of the literature, the probability of the diagnosis of SA increased with an increase in the synovial WBC count.Reference Margaretten, Kohlwes, Moore and Bent 2 In a meta-analysis, the sensitivity was 56% and 19%, and the specificity was 90% and 99% for a cellularity of 50,000 and 100,000/mm3, respectively.Reference Carpenter, Schuur, Everett and Pines 4 These studies are heterogeneous and did not include, in a prospective manner, patients with a suspected SA. In our study, the usefulness of this parameter was minimal. The sensitivity of direct Gram stain of synovial fluid was 40% in our study, and, in a review of the literature, it varied between 29% and 65%.Reference Carpenter, Schuur, Everett and Pines 4 Its specificity, which has not previously been studied, was 100% in our study. Curiously, the presence of microcrystals in the synovial fluid did not decrease the probability of SA, because 24% of the patients with SA had microcrystals. A similar frequency (22 of 104, 21%) was shown in a recent study, and the presence of microcrystals did not exclude SA.Reference Papanicolas, Hakendorf and Gordon 13 Finally, the opinion of the clinician who has the basic biological results possibly even the macroscopic appearance of the synovial fluid but not the result of its analysis, is relevant but not consistent; SA was not the most probable diagnosis in 5/38 (13%) of the SA cases. This could be explained by an over-reliance on the diagnostic value of fever and elevated inflammatory markers.
Our study has important limitations. The first challenge in the methodology is to decide what to do with cases of SA when bacteriological samples are negative. The prevalence of this situation ranges from 7% to 35% with significant differences in large case series.Reference Kaandorp, Dinant and van de Laar 14 – Reference Gupta, Sturrock and Field 16 To exclude these cases would lead to an underestimation of the frequency of SA. Second, none of the diagnostic criteria have been validated in adults; therefore, a pragmatic definition was adopted to include both bacteriological positive and negative SA as defined as: SA was considered probable when the clinician retained the diagnosis and treated accordingly, with antibiotic therapy for at least 3 weeks. With this definition, 24% of the patients had SA without bacteriological documentation. The analysis performed following the exclusion of these patients did not modify the study results. Given the large diversity at presentation, it is also challenging to define suspected SA. Painful swelling of the joint does not apply to deep joints. Acute monoarthritis does not apply to either 10% of SA cases, which involve more than one joint,Reference Dubost, Fis and Denis 17 or to a subacute or chronic presentation. Limiting the cases to those where a rheumatologist was contacted for a suspicion of SA was a pragmatic way to define suspected SA. When using these criteria, the prevalence of SA, which is the pretest probability, was 36% for those with suspected SA and 28% for bacteriologically documented SA. These were similar prevalence estimates to the pretest probability of bacteriologically proven SA at 21% and 27% in two prior studies.Reference Jeng, Wang and Liu 18 , Reference Talebi-Taher, Shirani, Nikanjam and Shekarabi 19
Conclusion
No clinical sign or non-bacteriological test, when taken alone, appears to be of value for the differentiation of SA from non-SA pathology, but the association of several factors, notably chills, the lack of any history of crystal-induced arthritis, radiological findings compatible with SA, and the appearance and cellularity of synovial fluid may combine to be suggestive as demonstrated in the two logistic regression models (AUC 0.85 and 0.87). In anticipation of having reliable markers of infection, this could serve to derive a score in patients with a suspected SA in order to estimate the diagnostic probability of SA and guide decisions on hospitalization or prescription of antibiotics. Both of these models require prospective validation prior to implementation.
Acknowledgements
The author wishes to thank Drs. Marielle Vayssade, Sandrine Mallochet-Guinamand, Anne Tournadre, Baptiste Glace, and Zuzana Tatar for their collection of data.
Competing interests: None declared.





