Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Carew, Evelyn Owen
Cooke, Francis W.
Lemons, Jack E.
Ratner, Buddy D.
Vesely, Ivan
and
Vogler, Erwin
1996.
Biomaterials Science.
p.
23.
Grasso, D.
Smets, B. F.
Strevett, K. A.
Machinist, B. D.
van Oss, C. J.
Giese, R. F.
and
Wu, W.
1996.
Impact of Physiological State on Surface Thermodynamics and Adhesion of Pseudomonas aeruginosa.
Environmental Science & Technology,
Vol. 30,
Issue. 12,
p.
3604.
Li, Z.
Giese, R. F.
Wu, W.
Sheridan, M. F.
and
van Oss, C. J.
1997.
THE SURFACE THERMODYNAMIC PROPERTIES OF SOME VOLCANIC ASH COLLOIDS.
Journal of Dispersion Science and Technology,
Vol. 18,
Issue. 3,
p.
223.
Azeredo, Joana
Ramos, Ilia
Rodriguks, Ligia
Oliveira, Rosrio
and
Teixeira, Jos
1997.
YEAST FLOCCULATION: A NEW METHOD FOR CHARACTERISING CELL SURFACE INTERACTIONS.
Journal of the Institute of Brewing,
Vol. 103,
Issue. 6,
p.
359.
Khayat, C.
Vatelot, A.
Decloux, M.
and
Bellon-Fontaine, M.N.
1997.
Evaluation of physico-chemical interactions in cross-flow filtration in the particular case of mineral membranes and sugar remelts.
Journal of Membrane Science,
Vol. 137,
Issue. 1-2,
p.
219.
Van Oss, C. J.
Giese, R. F.
and
Wu, W.
1997.
On the Predominant Electron-Donicity of Polar Solid Surfaces.
The Journal of Adhesion,
Vol. 63,
Issue. 1-3,
p.
71.
Sousa, M.
Azeredo, J.
Feijó, J.
and
Oliveira, R.
1997.
Polymeric supports for the adhesion of a consortium of autotrophic nitrifying bacteria.
Biotechnology Techniques,
Vol. 11,
Issue. 10,
p.
751.
Liu, Yue
and
Nancollas, George H.
1997.
Crystallization and Colloidal Stability of Calcium Phosphate Phases.
The Journal of Physical Chemistry B,
Vol. 101,
Issue. 18,
p.
3464.
van Oss, Carel Jan
1997.
Hydrophobicity and hydrophilicity of biosurfaces.
Current Opinion in Colloid & Interface Science,
Vol. 2,
Issue. 5,
p.
503.
Good, Robert J.
Islam, Mojahedul
Baier, Robert E.
and
Meyer, Anne E.
1998.
THE EFFECT OF SURFACE HYDROGEN BONDING (ACID-BASE INTERACTION) ON THE HYDROPHOBICITY AND HYDROPHILICITY OF COPOLYMERS: VARIATION OF CONTACT ANGLES AND CELL ADHESION AND GROWTH WITH COMPOSITION.
Journal of Dispersion Science and Technology,
Vol. 19,
Issue. 6-7,
p.
1163.
Dourado, F.
Gama, F.M.
Chibowski, E.
and
Mota, M.
1998.
Characterization of cellulose surface free energy.
Journal of Adhesion Science and Technology,
Vol. 12,
Issue. 10,
p.
1081.
van Oss, Carel J.
1998.
CURRICULUM VTTAE.
Journal of Dispersion Science and Technology,
Vol. 19,
Issue. 6-7,
p.
699.
Jin, Yu-Lai
and
Alex Speers, R.
1998.
Flocculation of Saccharomyces cerevisiae.
Food Research International,
Vol. 31,
Issue. 6-7,
p.
421.
Bardot, F.
Villiéras, F.
Michot, L.J.
Francois, M.
Gérard, G.
and
Cases, J.M.
1998.
HIGH RESOLUTION GAS ADSORPTION STUDY ON ILLITES PERMUTED WITH VARIOUS CATIONS ASSESSMENT OF SURFACE ENERGETIC PROPERTIES.
Journal of Dispersion Science and Technology,
Vol. 19,
Issue. 6-7,
p.
739.
Naim, J.O
van Oss, C.J
Ippolito, K.M.L
Zhang, J.-W
Jin, L.-P
Fortuna, R
and
Buehner, N.A
1998.
In vitro activation of human monocytes by silicones.
Colloids and Surfaces B: Biointerfaces,
Vol. 11,
Issue. 1-2,
p.
79.
Wu, Ŵeénju
and
Nancollas, George H.
1998.
THE FLOCCULATION OF AQUEOUS SUSPENSIONS OF TITANIA INDUCED BY SURFACE NUCLEATION OF CALCIUM PHOSPHATES#.
Journal of Dispersion Science and Technology,
Vol. 19,
Issue. 6-7,
p.
761.
Wiegel, D
Kaufmann, J
and
Arnold, K
1999.
Polar interactions of chondroitinsulfate.
Colloids and Surfaces B: Biointerfaces,
Vol. 13,
Issue. 3,
p.
143.
Wu, Wenju
and
Nancollas, George H.
1999.
Determination of interfacial tension from crystallization and dissolution data: a comparison with other methods.
Advances in Colloid and Interface Science,
Vol. 79,
Issue. 2-3,
p.
229.
van Oss, C. J.
Naim, J. O.
Costanzo, P. M.
Giese, R. F.
Wu, W.
and
Sorling, A. F.
1999.
Impact of Different Asbestos Species and Other Mineral Particles on Pulmonary Pathogenesis.
Clays and Clay Minerals,
Vol. 47,
Issue. 6,
p.
697.
Hermansson, Malte
1999.
The DLVO theory in microbial adhesion.
Colloids and Surfaces B: Biointerfaces,
Vol. 14,
Issue. 1-4,
p.
105.
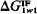 exactly zero. When
exactly zero. When 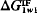 is positive, the interaction of the material with water dominates and the surface of the material is hydrophilic; when
is positive, the interaction of the material with water dominates and the surface of the material is hydrophilic; when 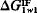 is negative, the polar cohesive attraction between the water molecules dominates and the material is hydrophobic. Thus, the sign of
is negative, the polar cohesive attraction between the water molecules dominates and the material is hydrophobic. Thus, the sign of 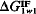 defines the nature of the surface and the magnitude of
defines the nature of the surface and the magnitude of 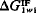 may be used as the natural quantitative measure of the surface hydrophobicity or hydrophilicity.
may be used as the natural quantitative measure of the surface hydrophobicity or hydrophilicity.