Introduction
Bipolar I disorder is a relatively common disorder (worldwide prevalence ~1%) characterized by a high degree of chronicity with recurrent major depressive episodes predominating over manic or hypomanic episodes during the symptomatic phase of illness.Reference Merikangas, Akiskal and Angst 1 -Reference Judd, Akiskal and Schettler 3 The chronicity of the illness is associated with a level of functional impairment that results in bipolar disorder being ranked among the top 20 causes of disability worldwide.Reference Vos, Flaxman and Naghavi 4 , Reference Forte, Baldessarini, Tondo, Vázquez, Pompili and Girardi 5
The long-term course of bipolar disorder is associated with an 8 to 15-year mean reduction in life expectancy compared to the general population.Reference Crump, Sundquist, Winkleby and Sundquist 6 -Reference Pan, Yeh, Chan and Chang 9 The higher mortality rate is attributable both to higher rates of suicide and higher rates of mortality due to medical illness.Reference Hayes, Miles, Walters, King and Osborn 8 , Reference Pan, Yeh, Chan and Chang 9 Specifically, individuals with bipolar disorder have higher rates of obesity, hypertension, and diabetes mellitus compared to the general population.Reference Wildes, Marcus and Fagiolini 10 -Reference Sayuri Yamagata, Brietzke, Rosenblat, Kakar and McIntyre 13 This constellation of metabolic abnormalities; central adiposity, dyslipidemia, hypertension, and hyperglycemia, known as metabolic syndrome (MetS) is associated with an increased risk of cardiovascular disease and type 2 diabetes.Reference Wilson, D’Agostino, Parise, Sullivan and Meigs 14 MetS is common in patients with mood disorders. The results of one meta-analysis reported a pooled MetS prevalence of 31.7% for bipolar disorder (N = 5827) and a pooled MetS prevalence of 31.3% for unipolar major depression (N = 5415).Reference Vancampfort, Vansteelandt and Correll 15
There is mounting evidence to suggest that cardiovascular disease and depressive disorders may share several underlying pathophysiological mechanisms, most notably high levels of inflammation (eg, inflammatory cytokines and high-sensitivity-CRP),Reference Munkholm, Vinberg and Vedel Kessing 16 -Reference Kessing, Ziersen, Andersen and Vinberg 18 high levels of oxidative stress markers,Reference Siwek, Sowa-Kućma and Dudek 19 , Reference Vaváková, Ďuračková and Trebatická 20 and an elevated level of autonomic dysfunction.Reference Kemp, Quintana, Gray, Felmingham, Brown and Gatt 21 , Reference Vinik and Ziegler 22 Development of MetS may also be exacerbated by treatments for bipolar disorder with certain atypical antipsychotic agents and mood stabilizers which can significantly increase the risk of developing obesity, insulin resistance, and dyslipidemia.Reference Bowden, Calabrese and McElroy 23 -Reference Vancampfort, Stubbs and Mitchell 27 However, it should be noted that the increased risk of metabolic syndrome in bipolar depression has been shown to occur independent of treatment with psychiatric medications.Reference Goldstein, Carnethon and Matthews 17 , Reference Guha, Bhowmick, Mazumder, Ghosal, Chakraborty and Burman 28
Onset of bipolar disorder occurs in childhood or adolescence in the majority of individuals,Reference Perlis, Miyahara and Marangell 29 and the pathogenesis of vascular and metabolic abnormalities has a similarly early onset in this population,Reference Goldstein, Carnethon and Matthews 17 so much so that a scientific statement from the American Heart Association has warned that “…bipolar disorder predispose(s) youth to accelerated atherosclerosis and early cardiovascular disease” (see also the “Call to Action” of the Vascular Task Force of the International Society for Bipolar Disorders).Reference Goldstein, Baune and Bond 30
Lurasidone is a second-generation antipsychotic agent that is approved in the US (and in several other countries) for the treatment of bipolar depression in adults and adolescents (age 10 to 17) as monotherapy, and as adjunctive therapy with lithium or valproate in adults. Evidence for the safety and efficacy of lurasidone in the treatment of bipolar depression is based on a series of short-termReference Loebel, Cucchiaro and Silva 31 -Reference Kato, Ishigooka and Miyajima 34 and long-term studies.Reference Ketter, Sarma, Silva, Kroger, Cucchiaro and Loebel 35 -Reference Pikalov, Tsai, Mao, Silva, Cucchiaro and Loebel 38
Lurasidone is recommended as a first-line treatment in adults with bipolar I depression in international guidelinesReference Fountoulakis 39 -Reference Yatham 41 and is the only first-line treatment recommended in pediatric patients with bipolar depression.Reference Yatham 41 Lurasidone acts as an antagonist with high affinity for D2, 5-HT2A, and 5-HT7 receptors, and as a partial agonist with moderate to high affinity for 5-HT1A receptors.Reference Ishibashi, Horisawa and Tokuda 42 Lurasidone exhibits weak affinity for 5-HT2C receptors and no appreciable affinity for muscarinic M1 and histamine H1 receptors.Reference Ishibashi, Horisawa and Tokuda 42 Although the mechanism of action is not fully understood, there is accumulating preclinical evidence suggesting that lurasidone achieves its antidepressant effect via a mixed D2/5-HT7 receptor antagonist mechanism (as summarized in Okubo et alReference Okubo, Hasegawa, Fukuyama, Shiroyama and Okada 43).
The objective of this analysis was to assess the effect of treatment with lurasidone on the rates of MetS in adult patients with bipolar depression. The analyses include data from three short-term (6 weeks) and two longer-term lurasidone clinical trials.
Methods
The protocols for all studies in this pooled post hoc analysis were approved by an independent ethics committee or institutional review board and all studies were conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and with the ethical principles of the Declaration of Helsinki. All patients reviewed and signed an informed consent document explaining study risks and procedures prior to study entry.
Short-term studies
Individual patient data were analyzed from 3 similarly designed multicenter, randomized, 6-week, placebo-controlled, double-blind studies of lurasidone for the treatment of bipolar depression in adults. These studies included: (a) a flexible-dose study evaluating monotherapy lurasidone (20–60 mg/day) (N = 166), lurasidone (80–120 mg/day) (N = 169), or placebo (N = 170) (NCT00868699),Reference Loebel, Cucchiaro and Silva 32 (b) a flexible-dose study evaluating lurasidone (20–120 mg/day) (N = 183) or placebo (N = 165) combined with therapeutic-level dosing with either lithium or valproate (NCT00868452),Reference Suppes, Kroger, Pikalov and Loebel 33 and (c) a flexible-dose study evaluating lurasidone (20–120 mg/day) (N = 180) or placebo (N = 176) combined with therapeutic-level dosing with either lithium or valproate (NCT01284517).Reference Kato, Ishigooka and Miyajima 34
All patients who entered the short-term trials were ages 18–75, met DSM-IV-TR criteria 44 for bipolar disorder, and currently experiencing a major depressive episode with Montgomery-Åsberg Depression Rating Scale (MADRS)Reference Montgomery and Åsberg 45 score ≥ 20 and a Young Mania Rating Scale (YMRS)Reference Biggs, Ziegler and Meyer 46 score ≤ 12. Key exclusion criteria were an acute or unstable medical condition, alcohol or other drug abuse (past 3 months) or dependence (past 12 months), imminent risk of suicide, or history of nonresponse to 3 or more adequate trials of an antidepressant during the current depressive episode.
Safety assessments included vital signs and laboratory assessments obtained on all patients at baseline and week 6 (or last visit).
Long-term studies
The long-term studies included: (a) a 6-month open-label lurasidone (20–120 mg/day) extension study that enrolled patients who completed one of the three 6-week double-blind studies (NCT00868699),Reference Ketter, Sarma, Silva, Kroger, Cucchiaro and Loebel 35 (b) a multicenter (26 sites in the U.S., South America, Europe, and Asia) open-label lead-in study in which patient received 12 to 20 weeks of lurasidone (20–120 mg/day) combined with lithium or valproate during an initial stabilization phase (NCT01358357),Reference Calabrese, Pikalov, Streicher, Cucchiaro, Mao and Loebel 36 and (c) a double-blind maintenance phase that enrolled clinically stabilized patients who completed the lead-in study and were randomized to continue adjunctive lurasidone (20–120 mg/day) in combination with lithium or valproate, or switch to placebo (in combination with lithium or valproate), for 28 weeks.Reference Calabrese, Pikalov, Streicher, Cucchiaro, Mao and Loebel 36
For the Calabrese et al study,Reference Calabrese, Pikalov, Streicher, Cucchiaro, Mao and Loebel 36 patients who met DSM-IV-TR criteria for bipolar I disorder were enrolled if they had 1 or more manic, mixed manic, or depressed episodes in the past 2 years and a current YMRS or MADRS total score of 14 or greater (if treated with lithium or valproate at the time of the screen visit) or 18 or greater (if not on lithium or valproate). Key exclusion criteria were the same as for the short-term studies. Safety assessment included vital signs and laboratory tests obtained at open-label baseline and endpoint in the Ketter et al study,Reference Ketter, Sarma, Silva, Kroger, Cucchiaro and Loebel 35 and baseline, end of 12–20-week lead-in phase, and end of 28-week double-blind phase in the Calabrese et al.Reference Calabrese, Pikalov, Streicher, Cucchiaro, Mao and Loebel 36
Classification of metabolic syndrome
Patients were classified as having metabolic syndrome based on the 2005 revision of the NCEP ATP III criteriaReference Grundy, Cleeman and Daniels 47 when any 3 of the following 5 criteria were met: elevated waist circumference (≥102 cm for men, ≥88 cm for women), elevated triglycerides (≥150 mg/dL), reduced high-density lipoprotein cholesterol (<40 mg/dL in men, <50 mg/dL in women), elevated blood pressure (systolic ≥130 mm Hg or diastolic ≥85 mm Hg), or elevated fasting glucose (≥100 mg/dL).
Statistical analysis
For the short-term studies, the analysis population consisted of patients who were randomly assigned to treatment, received ≥1 dose of study medication, and had a baseline assessment for metabolic syndrome. For the open-label Ketter et al. studyReference Ketter, Sarma, Silva, Kroger, Cucchiaro and Loebel 35 and lead-in phase of the Calabrese et al. study,Reference Calabrese, Pikalov, Streicher, Cucchiaro, Mao and Loebel 36 the analysis population consisted of all patients who received ≥1 dose of open-label study medication and had a baseline assessment for metabolic syndrome. The analysis population for the 28-week double-blind phase of the Calabrese et al studyReference Ishigooka, Kato and Miyajima 37 comprised all patients enrolled in that phase who received at least one dose of double-blind study medication and had endpoint (post baseline of the 28 week phase) data for determining metabolic syndrome classification.
For both the short and long-term studies, the percent of patients who had MetS at baseline and endpoint (observed case analysis [OC] or last observation carried forward [LOCF]) was calculated for each treatment group. In addition, the percent of patients who had new onset MetS (ie, did not qualify for MetS at baseline but did qualify at endpoint) was calculated. Finally, the proportion of patients with clinically significant weight gain or weight loss (≥7%) was calculated; number needed to harm (NNH) values were also calculated for the placebo-controlled studies.
Results
Pooled analysis of short-term studies
In the pooled analysis of the 3 double-blind, placebo-controlled, short-term studies, there were 1209 randomized patients with bipolar I depression, of whom 1192 patients received at least one dose of study medication and had a baseline assessment with metabolic data. In this group, a total of 941 (78.9%) were study completers; of these 1192 patients, 330 were treated with lurasidone monotherapy, 168 with placebo monotherapy, 360 with lurasidone adjunctive with lithium or valproate, and 334 with placebo adjunctive with lithium or valproate. For the pooled sample overall, 46.7% were male, 64% were white, and the mean age was 42.2 years (Table 1). The mean (SD) duration of the current depressive episode was 2.98 (2.2) months. Based on the NCEP ATP III criteria for each parameter, abnormal values were observed in 41% of patients for waist circumference, 37% for HDL cholesterol, 34% for triglycerides, 32% for blood pressure, and 23% for glucose. Combining patients from all short-term studies, the overall rate of metabolic syndrome at baseline was 25.2% (300/1192).
Table 1. Baseline Clinical and Demographic Characteristics
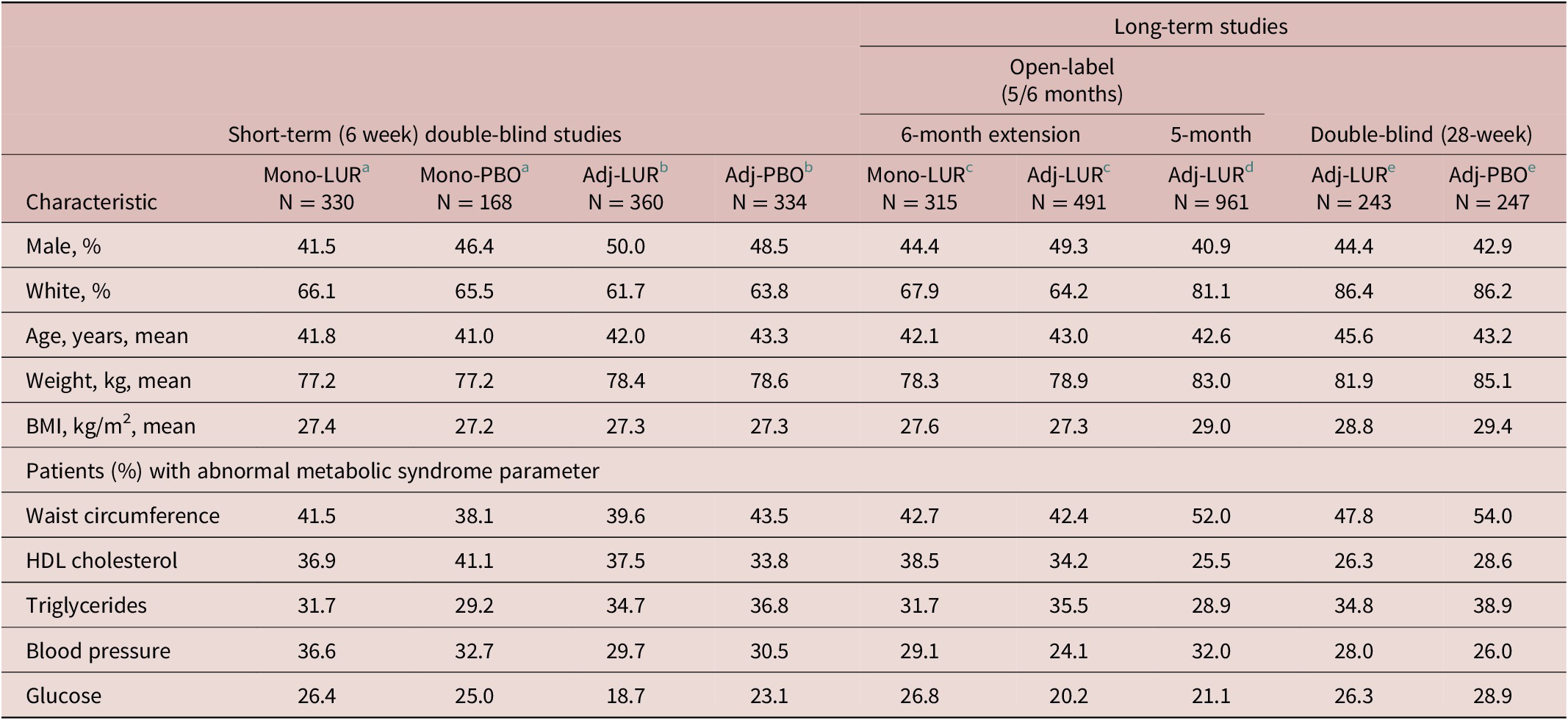
Note: Sample sizes vary based on data availability. Abnormal metabolic syndrome percentages based on NCEP ATP III criteria cutoff levels for each parameter. The sample sizes represent the number of patients in the safety population who had data available for individual metabolic syndrome parameters.
Abbreviations: Adj, adjunctive therapy; BMI, body mass index; HDL, high-density lipoprotein; Mono, monotherapy.
a Data from Loebel et al.Reference Loebel, Cucchiaro and Silva 31
b Pooled data from Loebel et alReference Loebel, Cucchiaro and Silva 32 and Suppes et al.Reference Suppes, Kroger, Pikalov and Loebel 33
c 6-month extension phase data from Ketter et alReference Ketter, Sarma, Silva, Kroger, Cucchiaro and Loebel 35 that pools patients recruited from the Loebel et al,Reference Loebel, Cucchiaro and Silva 31 Loebel et al,Reference Loebel, Cucchiaro and Silva 32 and Suppes et al.Reference Suppes, Kroger, Pikalov and Loebel 33
d Data from Calabrese et alReference Calabrese, Pikalov, Streicher, Cucchiaro, Mao and Loebel 36 initial stabilization phase of up to 5 months.
e Data from Calabrese et alReference Calabrese, Pikalov, Streicher, Cucchiaro, Mao and Loebel 36 double-blind 6-month maintenance phase.
Mean changes from baseline to week 6 (LOCF) on each of the individual metabolic syndrome (MetS) parameters were small for both the lurasidone monotherapy and the lurasidone adjunctive therapy studies (Table 2). At LOCF-endpoint the difference in the proportion of patients with clinically significant weight gain (≥7%) was small for both monotherapy lurasidone vs placebo (NNH = 59) and for adjunctive therapy (NNH = 36) (Table 2).
Table 2. Mean Change from Baseline in Metabolic Syndrome Criteria; and Mean Change in Weight, and Proportion with ≥ 7% Weight Gain
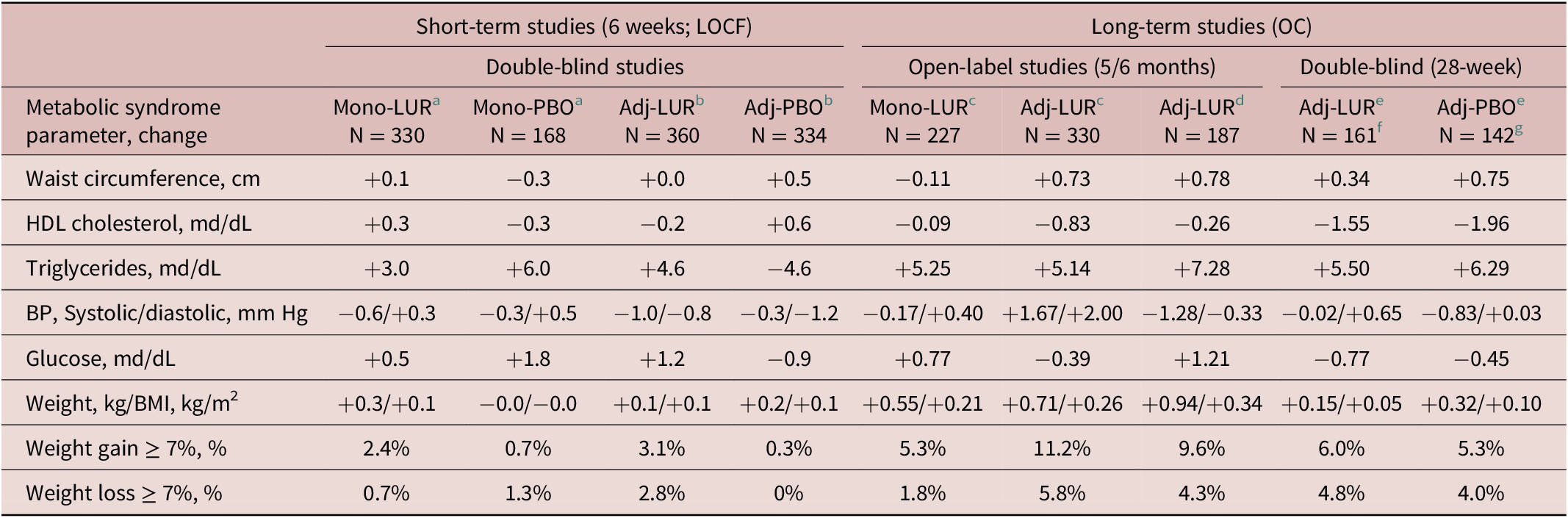
Note: For patients in the long-term studies, mean change was calculated from open-label baseline. The sample sizes represent the number of patients in the safety population who had data available for individual metabolic syndrome parameters in the last observation carried forward (LOCF) analyses for the short-term studies, and the observed case (OC) analyses for the long-term studies.
Abbreviations: Adj, adjunctive therapy; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LOCF, last observation carried forward; Mono, monotherapy; OC, observed case.
a Data from Loebel et al.Reference Loebel, Cucchiaro and Silva 31
b Pooled data from Loebel et alReference Loebel, Cucchiaro and Silva 32 and Suppes et al.Reference Suppes, Kroger, Pikalov and Loebel 33
c 6-month extension phase data from Ketter et alReference Ketter, Sarma, Silva, Kroger, Cucchiaro and Loebel 35 that pools patients recruited from the Loebel et al,Reference Loebel, Cucchiaro and Silva 31 Loebel et al,Reference Loebel, Cucchiaro and Silva 32 and Suppes et al.Reference Suppes, Kroger, Pikalov and Loebel 33
d Data from Calabrese et alReference Calabrese, Pikalov, Streicher, Cucchiaro, Mao and Loebel 36 initial stabilization phase of up to 5 months.
e Data from trials of Calabrese et alReference Calabrese, Pikalov, Streicher, Cucchiaro, Mao and Loebel 36 double-blind 6-month maintenance phase.
f Weight/BMI, n = 167.
g weight/BMI, n = 150.
At baseline, the proportion of patients meeting criteria for MetS in the monotherapy and adjunctive therapy studies, respectively, were 27.6% and 23.6% for lurasidone, and 23.8% and 25.1% for placebo (Figure 1A). At week 6 (LOCF) the proportion meeting criteria for MetS in the monotherapy and adjunctive therapy studies, respectively, were 27.5% and 26.6%, for lurasidone, and 29.9% and 20.2% for placebo (Figure 1A).
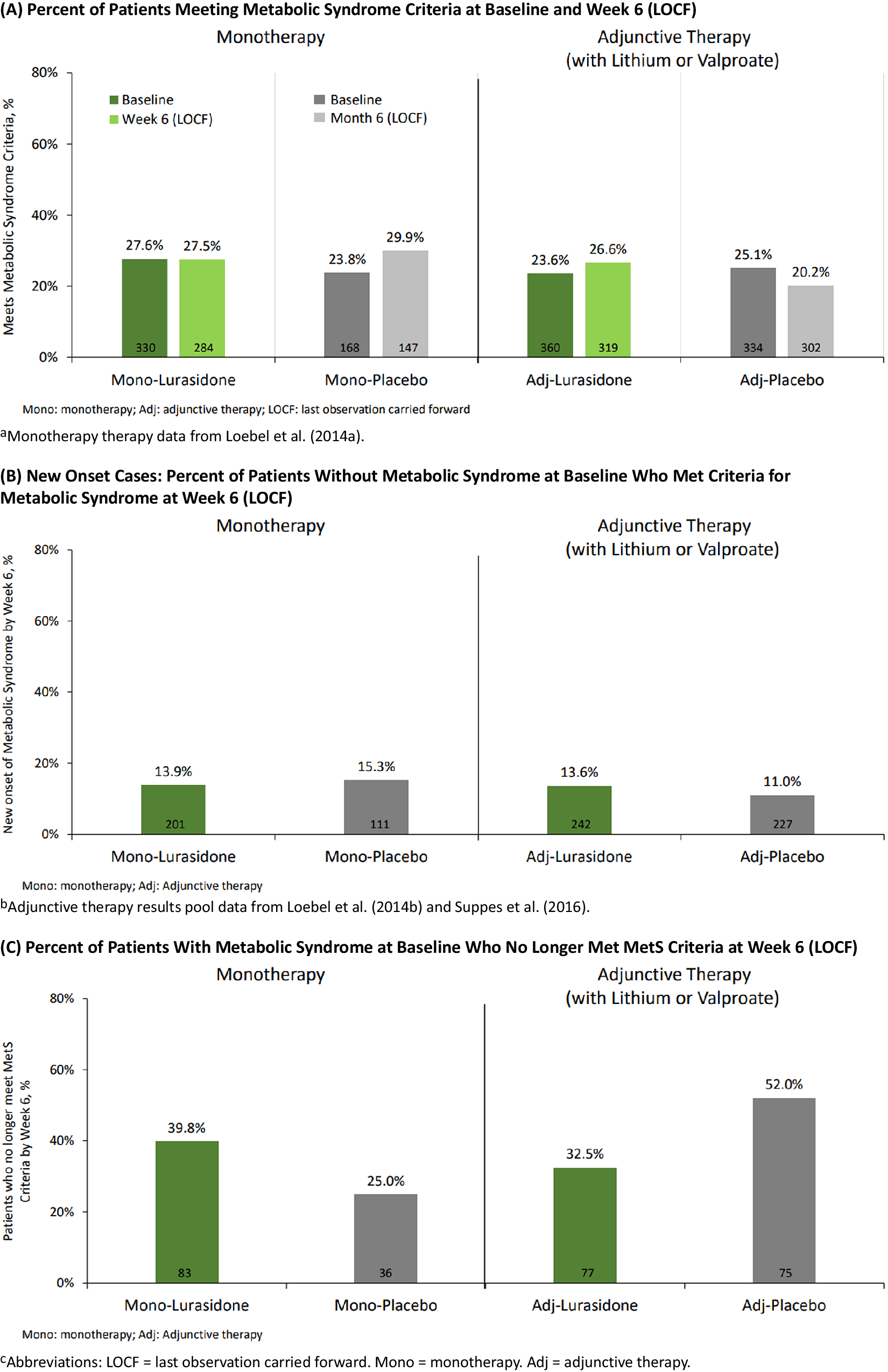
Figure 1. Metabolic syndrome status: short-term studies pooled. (A) Percent of patients meeting metabolic syndrome criteria at baseline and week 6 (LOCF). Monotherapy therapy data from Loebel et al.Reference Loebel, Cucchiaro and Silva 31. (B) New onset cases: percent of patients without metabolic syndrome at baseline who met criteria for metabolic syndrome at week 6 (LOCF). Adjunctive therapy results pool data from Loebel et alReference Loebel, Cucchiaro and Silva 32 and Suppes et al.Reference Suppes, Kroger, Pikalov and Loebel 33. (C) Percent of patients with metabolic syndrome at baseline who no longer met MetS criteria at week 6 (LOCF). Adj, adjunctive therapy; LOCF, last observation carried forward; Mono, monotherapy.
Among patients without MetS at baseline (and who had metabolic data available at Week 6), the proportion developing treatment-emergent MetS by week 6 (LOCF) was similar for monotherapy lurasidone vs placebo (13.9% vs 15.3%), and for adjunctive lurasidone vs placebo (13.6% vs 11.0%) (Figure 1B). Among patients with MetS at baseline, the proportion who no longer met MetS criteria at week 6 (LOCF) for monotherapy lurasidone vs placebo was: 39.8% vs 25.0%; and for adjunctive lurasidone vs placebo was: 32.5% vs 52.0% (Figure 1C).
Long-term studies
In the long-term study section of Table 1, the first two columns summarize the demographic and clinical characteristics of patients who had completed one of the short-term, double-blind studies and were entering the 6-month extension phase. As expected, patient characteristics at extension phase baseline were similar to the acute phase baseline characteristics (male, ~47%; white, ~65%; age, ~43% years; weight, ~79 kg; BMI, ~27 kg/m2). The third column of Table 1 shows baseline data for patients entering a 5-month open-label, stabilization phase (on adjunctive lurasidone) prior to being randomized to 28 weeks of double-blind treatment with either adjunctive lurasidone or placebo. Patients at the 5-month, open-label baseline were similar in age (42.6 years) to the group of patients who continued into the 28-week double-blind phase, but were somewhat more likely to be white (81.1%) females (59.1%) with a higher weight (83 kg) and BMI (29 kg/m2).
Table 2 summarizes the mean change in metabolic syndrome criteria during long-term treatment, based on an observed case (OC) analysis. At endpoint (OC) in the double-blind 7-month study, the proportion of patients with clinically significant weight gain (≥7%) was small for both adjunctive lurasidone and adjunctive placebo (NNH = 15) (Table 2). At endpoint (OC) in the 5–6-month open-label studies, the proportion with clinically significant weight gain was larger for patients in the 2 studies who were treated with adjunctive lurasidone (5.3% and 11.2%) compared to patients in the study who were treated with lurasidone monotherapy (1.8%).
The proportion of patients meeting criteria for MetS at open-label Baseline and endpoint (OC), respectively, was 28.6% and 27.7% for monotherapy lurasidone, 22.6% and 28.7% for adjunctive lurasidone in the 6-month open-label extension study; 24.7% and 24.7% for adjunctive lurasidone in the 5-month open-label study; and 23.5% and 28.0% for adjunctive lurasidone and 30.4% and 30.8% for adjunctive placebo in the 28-week double-blind study (Table 3-A).
Table 3. Metabolic Syndrome Status During Long-Term Studies
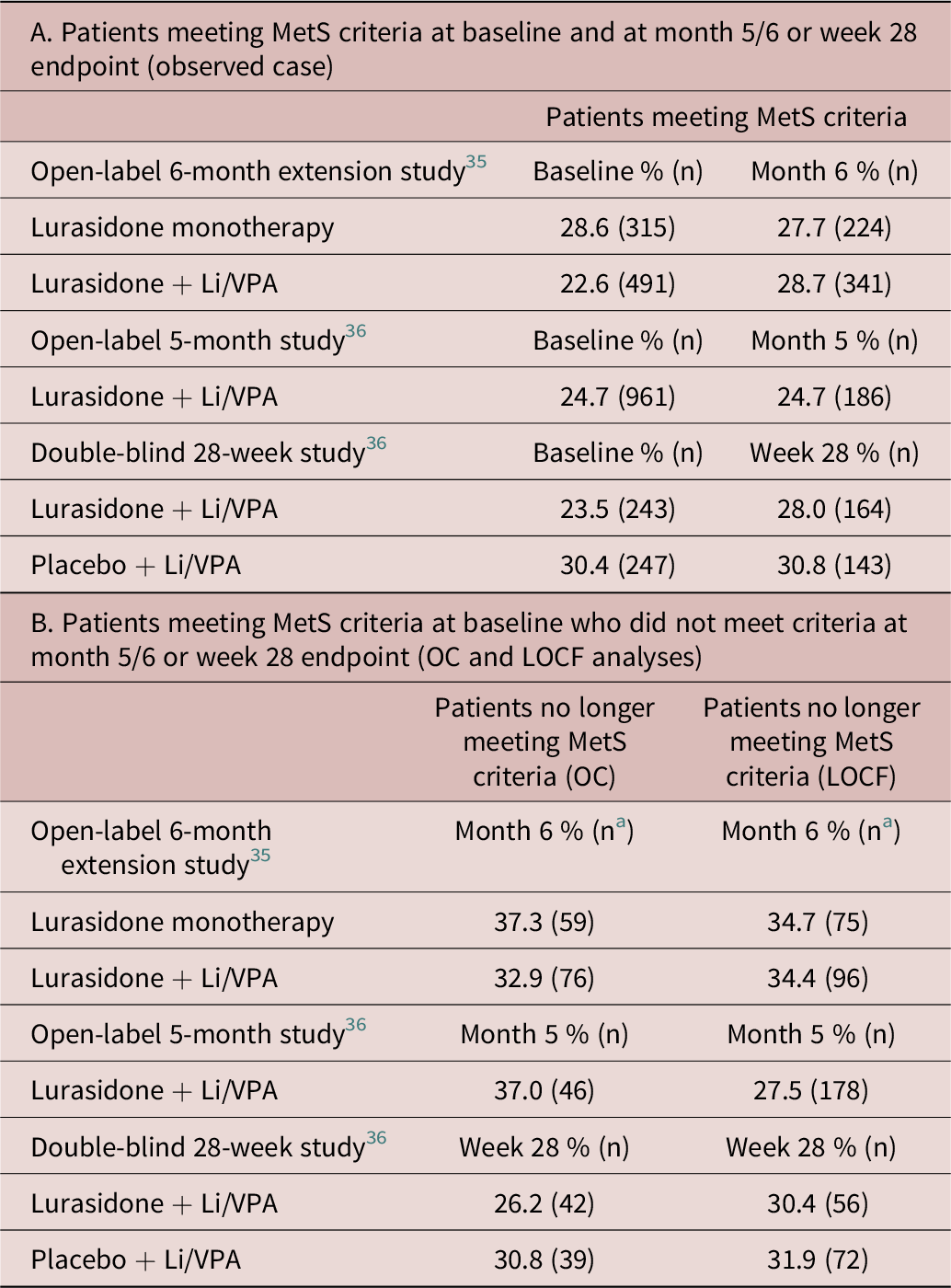
Abbreviations: LOCF, last observation carried forward; MetS, metabolic syndrome; OC, observed case.
a Number of subject who met MetS criteria at baseline and had MetS data available at month 6 (OC/LOCF).
The proportion of patients meeting criteria for MetS at open-label Baseline, but who did not meet criteria at endpoint was 37.3% (OC) and 34.7% (LOCF) for monotherapy lurasidone and 32.9% (OC) and 34.4% (LOCF) for adjunctive lurasidone in the 6-month open-label extension study; 37.0% (OC) and 27.5% (LOCF) for adjunctive lurasidone in the 5-month open-label study; and 26.2% (OC) and 30.4% (LOCF) for adjunctive lurasidone and 30.8% (OC) and 31.9% (LOCF) for adjunctive placebo in the 28-week double-blind study (Table 3-B).
Among patients in the three long-term studies without MetS at long-term Baseline, the proportion developing treatment-emergent MetS by endpoint (OC and LOCF) are summarized in Table 4. The results of the more clinically useful OC analyses found new-onset cases of MetS to occur at a consistently lower rate in the lower (40–60 mg/d) doses of lurasidone compared to the higher (80–120 mg/d) doses in the 6-month extension study (20.3% vs 11.8%), in the 5-month open-label study (14.1% vs 8.5%), and in the double-blind 28-week study (13.3% vs 6.9%; and vs 16.7% in the placebo group). A similar pattern of results was observed in the LOCF lurasidone dose analysis. In the 6-month and 5-month open-label studies in Table 4, the rate of new-onset MetS was approximately similar in patients treated with adjunctive lurasidone regardless of whether the adjunctive agent was lithium or VPA. However, in the double-blind 28-week study, new-onset cases were notably higher in patients treated with lurasidone adjunctive with lithium compared to VPA (20.4% vs 5.7%) but were much lower in placebo treated patients with lithium compared to VPA (7.9% vs 21.9%).
Table 4. New Onset Metabolic Syndrome: Proportion of Patients Without Metabolic Syndrome at Baseline Who Met Criteria for Metabolic Syndrome at Month 5/6 or Week 28 Endpoint
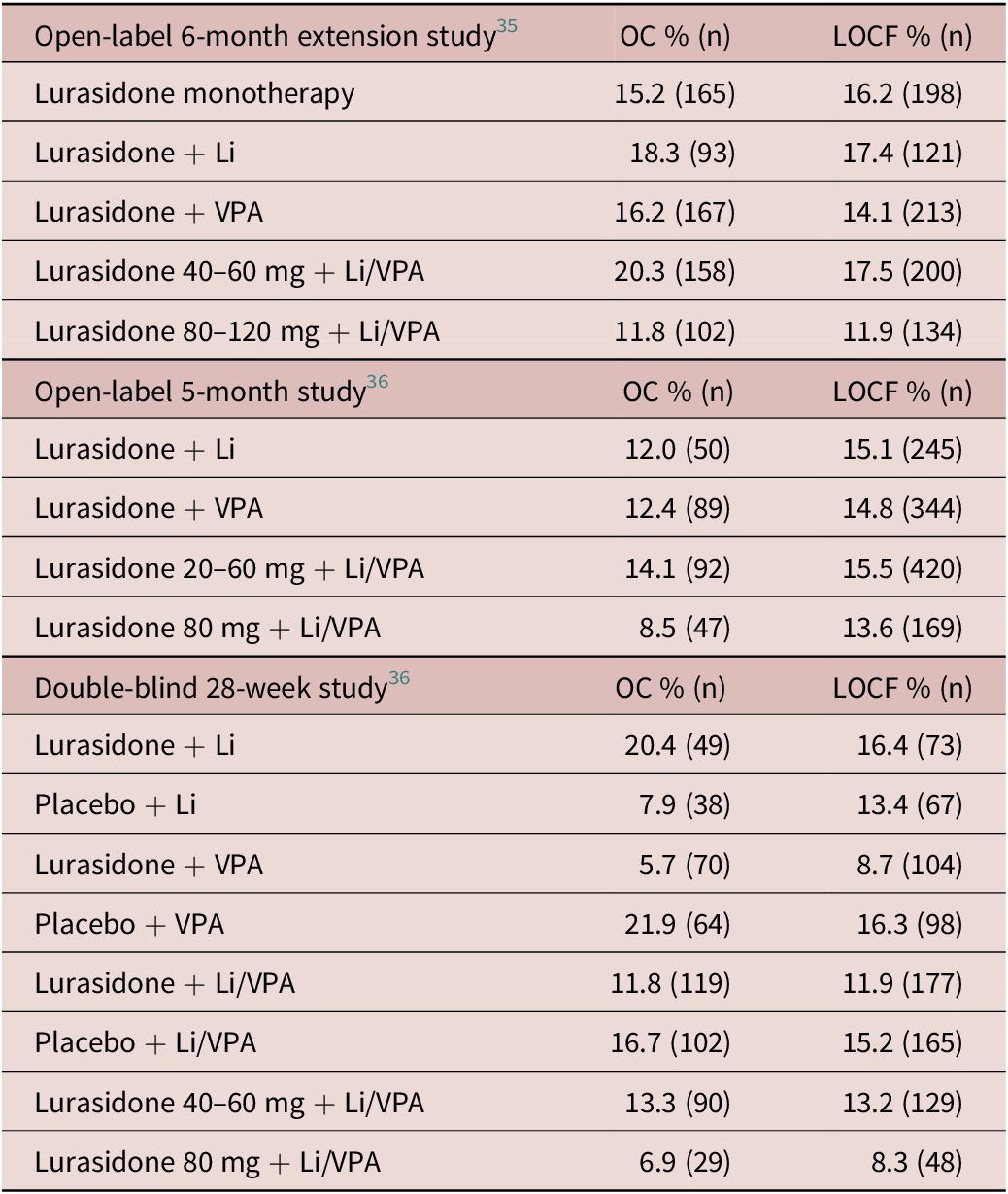
Abbreviation: OC, observed case; LOCF, last observation carried forward.
Discussion
This post hoc, pooled analysis demonstrated that short- and long-term, adjunctive or monotherapy with lurasidone in patients with bipolar depression was not associated with a significant increase in treatment-emergent metabolic syndrome. In comparisons of the 6-week double-blind treatment studies, the proportion of new-onset cases of MetS were minimally different for both adjunctive or monotherapy with lurasidone when compared to placebo, with differences that were neither statistically significant or clinically meaningful. Furthermore, no clinically meaningful mean changes in the components of MetS (waist circumference, triglycerides, high-density lipoprotein cholesterol, hypertension, or fasting glucose) occurred during short-term adjunctive or monotherapy with lurasidone. Similar results were obtained in the 5–6-month open-label adjunctive or monotherapy studies with lurasidone (range, 12–18%) and in the double-blind adjunctive therapy study (range, 6–20%). The new-onset MetS rates were offset by higher MetS offset rates—patients who met MetS criteria at Baseline, but who no longer met MetS criteria at 5–6-month endpoint—in the two open-label studies (range, 33–37%) and in the double-blind adjunctive therapy study (26%). In addition, no clinically meaningful mean changes in the components of MetS occurred during long-term adjunctive or monotherapy with lurasidone.
Taken together, these findings are consistent with previous reports from individual bipolar depression studies which found minimal effects of lurasidone on weight, lipids, and glycemic indices,Reference Loebel, Cucchiaro and Silva 32 -Reference Calabrese, Pikalov, Streicher, Cucchiaro, Mao and Loebel 36 and extend these previous results to the assessment of MetS. The results are also consistent with previous short and long-term studies of lurasidone in the treatment of schizophrenia, another disorder in which metabolic syndrome is a major clinical concern.Reference Ogasa, Kimura, Nakamura and Guarino 48 -Reference Citrome, Cucchiaro and Sarma 57
One notable finding was the consistently lower rate of new-onset MetS in patients treated with higher doses of lurasidone, regardless of whether treatment was adjunctive with lithium or VPA or monotherapy. The lack of any dose–response effect makes it more likely that lurasidone is metabolically neutral, and that fluctuations in MetS status is independent of lurasidone-specific treatment effects.
In a comparison of the metabolic effects of lithium vs VPA, rates of new-onset MetS were approximately similar in both the 6-month and 5-month open-label studies. However, the results for the double-blind 28-week study offer a confusing picture, with notably higher rates of new-onset MetS in the lurasidone/lithium group compared to the lurasidone/VPA group, but notably lower rates of new onset MetS in the placebo/lithium group compared to the placebo/VPA group. The clinical literature consistently shows that VPA is associated with greater weight and metabolic effects compared to lithium.Reference Hayes, Marston, Walters, Geddes, King and Osborn 58 The reason for the puzzling metabolic findings on lithium in the double-blind 28-week study is uncertain.
It should be noted that the current post hoc treatment sample exhibited a somewhat lower MetS prevalence rate at baseline in both the short-term and long-term lurasidone studies—25% and 27%, respectively—compared to the MetS prevalence rate of 31.7% (95% CI, 27.3%–36.3%) reported in a bipolar disorder meta-analysis.Reference Vancampfort, Vansteelandt and Correll 15 This is likely due to the entry criteria in the current studies that excluded patients with more severe “acute or unstable” components of MetS such as diabetes and hypertension. In addition, the MetS criteria utilized in the current analysis required that a patient meet specific criteria for elevated blood pressure, triglycerides, and glucose, while medication treatment for hypertension, hypertriglyceridemia, or diabetes was insufficient to qualify as a criterion without an abnormal lab value.
The high prevalence rate of MetS, together with accumulating evidence on the presence of inflammatory and oxidative stress markers associated with vascular endothelial damage in patients with bipolar depression,Reference Munkholm, Vinberg and Vedel Kessing 16 -Reference Vaváková, Ďuračková and Trebatická 20 have raised the possibility of shared pathophysiological processes for bipolar depression and endovascular disease.Reference Munkholm, Vinberg and Vedel Kessing 16 , Reference Goldstein, Carnethon and Matthews 17 , Reference Siwek, Sowa-Kućma and Dudek 19 For example, diabetes/hyperglycemia/insulin-resistance and hyperlipidemia have both been reported to be associated with an increased risk of affective illness.Reference Ali, Stone, Peters, Davies and Khunti 59 -Reference Vancampfort, Mitchell and De Hert 65
It is important to note that study entry criteria that excluded patients with clinically significant baseline abnormalities in fasting glucose, triglycerides, cholesterol, and blood pressure may reduce the generalizability of the current study results to patients in the community.
Regardless of the nature of the connection between bipolar depression and vascular disease, the increased risk of MetS, vascular morbidity, and associated reduction in life expectancy among individuals with a bipolar disorder diagnosis is an important consideration in clinical decision-making. This is especially true since maintenance therapy is often indicated in patients with bipolar disorder. The lack of adverse effects on weight, metabolic parameters, and risk of MetS in the current post hoc analyses of short and long-term studies in bipolar depression suggests that lurasidone may have a favorable risk–benefit profile in the treatment of this chronic and frequently disabling disorder.
Financial Support
Edward Schweizer of Paladin Consulting Group provided medical writing and editorial assistance for this manuscript that was funded by Sunovion Pharmaceuticals Inc., Marlborough, MA. Clinical research was sponsored by Sunovion Pharmaceuticals Inc. The sponsor was involved in the study design, collection, and analysis of data. The interpretation of results and the decision to submit this manuscript for publication were made by the authors independently.
Author Contributions
Conceptualization: M.T., J.W.N., Y.M., A.P.; Formal analysis: M.T., Y.M.; Methodology: M.T., J.W.N., Y.M., A.P.; Writing—original draft: M.T.; Writing—review and editing: M.T., J.W.N., Y.M., A.P.
Disclosures
M.T., Y.M., and A.P. are employees of Sunovion Pharmaceuticals Inc. J.W.N. has received grant support from the National Institutes of Health and the Substance Abuse and Mental Health Services Administration, has served as a consultant for Alkermes, Inc., Intra-cellular Therapies, Inc., and Sunovion, and served on a Data Safety Monitoring Board for Amgen.







