Take-Home Points
-
1. Vortioxetine has a complex mechanism of action that includes not only inhibition of serotonin (5HT) transporters (SERT), but also direct actions at multiple 5HT receptor subtypes (5HT1A, 5HT1B, 5HT1D, 5HT3, and 5HT7 receptors).
-
2. Vortioxetine has direct actions at 5HT1A and 5HT1B heteroreceptors that may explain in part how vortioxetine causes the downstream release of dopamine (DA), norepinephrine (NE), histamine (HA), and acetylcholine (ACh).
-
3. Enhanced release of neurotransmitters in the prefrontal cortex and hippocampus could hypothetically help to explain vortioxetine’s antidepressant actions and unique procognitive properties in patients with major depression.
Vortioxetine is a “multimodal” agent that simultaneously acts at 6 pharmacologic targets with 3 modes of action (Figure 1)Reference Mørk, Pehrson and Brennum 1 – Reference Mørk, Montezinho and Miller 5 :
-
1. Inhibition of the serotonin (5HT) transporter or SERT
-
2. Actions at several G-protein linked receptors (agonist actions at 5HT1A receptors, partial agonist actions at 5HT1B receptors, antagonist actions at 5HT1D and 5HT7 receptors)
-
3. Inhibition of a ligand-gated ion channel (the 5HT3 receptor)

Figure 1 Icon of vortioxetine showing its 6 pharmacologic mechanisms. Highlighted here are 5HT1A agonism and 5HT1B partial agonism, potentially linked to vortioxetine’s actions of enhancing the release of NE, DA, ACh, and HA.
We have previously described the mechanisms whereby vortioxetine’s actions at 5HT receptors work together to enhance the release of 5HT,Reference Stahl 6 glutamate,Reference Stahl 7 acetylcholine (ACh),Reference Stahl 8 and norepinephrine (NE)Reference Stahl 8 and to inhibit the release of GABA (gamma amino butyric acid).Reference Stahl 7 Here we discuss how the interaction of vortioxetine at populations of 5HT1A and 5HT1B receptors (Figure 1) may also hypothetically contribute to the release of ACh and NE and furthermore lead to release of dopamine (DA) and histamine (HA) in the prefrontal cortex (Figures 2 and 3). Enhanced release of neurotransmitters may theoretically “tune” malfunctioning brain circuits.Reference Stahl 6 – Reference Stahl 11 Specifically, enhanced release of 5HT, NE, DA, ACh, and HA by vortioxetine could theoretically improve the efficiency of information processing in maladaptive brain circuits by facilitating long-term potentiation, synaptic plasticity, and enhanced pyramidal neuron activity leading to improvement not only of mood but also of cognitive symptoms in major depressive disorder.

Figure 2A Possible regulation of NE, DA, and ACh release by cortical postsynaptic serotonin 1A heteroreceptors. GABA release is inhibited by 5HT1A input to GABAergic interneurons that in turn innervate the presynaptic nerve terminals of NE, DA, and ACh neurons.
Improving the Efficiency of Information Processing in Neuronal Networks by Enhancing the Release of Key Neurotransmitters
In this series of articles on the mechanism of action of vortioxetine, we have discussed how the many modes of action of this agent interact with multiple 5HT receptor subtypes localized at various critical nodes that connect 5HT neurons to a network of several other neurons.Reference Stahl 6 – Reference Stahl 8 Actions of neurotransmitters, drugs, and psychiatric illnesses can be understood not only within “microcircuits”—eg, connections between a presynaptic 5HT neuron and a postsynaptic site—but also within “macrocircuits,” eg, where serotonergic neurons are part of a neuronal network that connects many neurons with each other.Reference Stahl 6 – Reference Pehrson and Sanchez 13 The sites where neurons connect with each other are also called the “nodes” of a neuronal network, and in the case of 5HT nodes, are linked by many different 5HT receptor subtypes. Not only does 5HT acting at these nodes regulate its own release, but it modulates the release of every major neurotransmitter.Reference Mørk, Pehrson and Brennum 1 – Reference Stahl 8 , Reference Fink and Gothert 12 , Reference Pehrson and Sanchez 13 We have discussed how this happens with 5HT itself at 5HT neurons regulated by numerous autoreceptors,Reference Stahl 6 with glutamate and GABA at connecting neurons receiving 5HT input especially at many different 5HT receptors,Reference Stahl 7 and with ACh, NE, and 5HT via actions at 5HT3 receptors.Reference Stahl 8 Here we describe 2 additional ways in which 5HT could theoretically modulate neurotransmitter release at 5HT1A and 5HT1B heteroreceptors in the prefrontal cortex, and specifically how vortioxetine acting at these sites could potentially enhance the release of DA, NE, ACh, and HA (Figures 2 and 3).
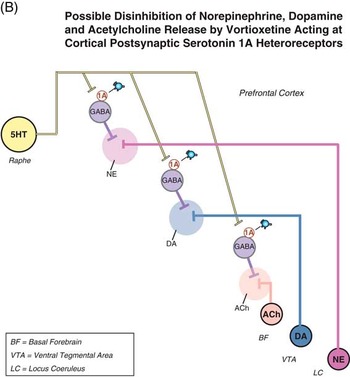
Figure 2B Possible disinhibition of NE, DA, and ACh release by vortioxetine acting at cortical postsynaptic serotonin 1A heteroreceptors. Vortioxetine directly stimulates 5HT1A receptors on GABA interneurons innervating the presynaptic nerve terminals of NE, DA, and ACh neurons. This could potentially disinhibit (enhance) the release of DA, NE, and ACh from their nerve terminals in the prefrontal cortex.
5HT1A Postsynaptic Heteroreceptors in Prefrontal Cortex: Potential Modulation of DA, ACh, and NE Release
We have previously discussed the role of 5HT1A presynaptic autoreceptors located in the midbrain raphe and on soma and dendrites of 5HT neurons.Reference Stahl 6 These receptors desensitize over time when either SSRIs or vortioxetine are given, leading to disinhibition of 5HT release.Reference Assié, Lomentach, Ravailhe, Faucillon and Newman-Tancredi 14 – Reference Sprouse and Aghajanian 17 By contrast, when these same 5HT1A receptors are localized on postsynaptic sites, they do not seem to desensitize over time after the administration of SSRIs or vortioxetine, but they regulate the release of many other neurotransmitters.11,14–17 5HT1A postsynaptic receptors are inhibitory, so stimulating them either indirectly after SERT blockade by an SSRI or directly with a 5HT1A agonist such as vortioxetine will inhibit the firing of that neuron.Reference Stahl 11 , Reference Assié, Lomentach, Ravailhe, Faucillon and Newman-Tancredi 14 – Reference Sprouse and Aghajanian 17 We have already discussed how this specifically inhibits certain GABA interneurons regulating glutamate release.Reference Stahl 7 , Reference Stahl 8 Here we discuss and illustrate the possible actions of 5HT at a different population of GABA neurons in prefrontal cortex, namely, those that possibly regulate the release of NE, DA, and ACh at their presynaptic nerve terminals (Figure 2A). GABA release is inhibited by 5HT1A input to these GABAergic interneurons, so when vortioxetine stimulates these 5HT1A receptors, this could potentially disinhibit the release of ACh,Reference Izumi, Washizuka, Miura, Hiraga and Ikeda 18 , Reference Consolo, Ramponi, Ladinsky and Baldi 19 NE,Reference Suzuki, Matsuda, Asano, Somboonthum, Takuma and Baba 20 , Reference Suwabe, Kubota, Niwa, Kobayashi and Kanba 21 and DAReference Díaz-Mataix, Scorza, Bortolozzi, Toth, Celada and Artigas 22 , Reference Alex and Pehak 23 from their nerve terminals in the prefrontal cortex (Figure 2B). Not shown because of the lack of data—but still a theoretical possibility—is the same regulatory system for histamine. Figures 2A and 2B are just hypothetical wiring diagrams that are consistent with the available data, but require much further investigation to confirm that this is how 5HT1A agonists actually increase ACh, NE, and DA release.
5HT1B Postsynaptic Heteroreceptors in the Prefrontal Cortex: Potential Modulation of DA, ACh, NE, and HA Release
We have also previously discussed the role of 5HT1B autoreceptors that are located on 5HT nerve terminals and inhibit 5HT release.Reference Stahl 6 When 5HT occupies these receptors, it inhibits further 5HT release from those 5HT nerve terminals.Reference Stahl 6 , Reference Stahl 11 , Reference Fink and Gothert 12 To the extent that vortioxetine blocks these 5HT1B receptors as a partial agonist or functional antagonist, the opposite effect occurs, namely enhanced 5HT release, as discussed previously.Reference Stahl 6 We have also previously discussed the possible role of 5HT1B heteroreceptors that may be localized on some GABAergic interneurons and may regulate GABA release.Reference Stahl 7
Here, however, we discuss and illustrate the actions of 5HT at a different 5HT1B receptor that is possibly localized directly upon the nerve terminals of neurons that release NE, DA, ACh, and HA (Figure 3A).Reference Fink and Gothert 12 , Reference Maura and Raiteri 24 , Reference Maura, Fedele and Raiteri 25 Although the microanatomy is still being worked out, blockade of postsynaptic 5HT1B heteroreceptors on presynaptic nerve terminals could theoretically be another mechanism whereby ACh, NE, DA, and HA release is enhanced by vortioxetine (Figure 3B).Reference Stahl 8 , Reference Stahl 10 This is somewhat speculative and is not yet a proven mechanism for regulating the release of these neurotransmitters, but is consistent with the known effects of vortioxetine enhancing the release of ACh, NE, DA, and HA. Few if any other agent has the broad neurotransmitter-releasing properties of vortioxetine, and a full explanation of the mechanism of this release will require a more complete clarification of the various circuits involving 5HT receptors and other neurotransmitter systems. For example, some evidence suggests that stimulation of 5HT4 receptors may be in part responsible for 5HT’s regulation of HA and ACh release.Reference Johnson, Drummond and Grimwood 26 For now, mechanisms such as the one illustrated in Figure 3B remain only theories as to how vortioxetine could potentially disinhibit the release of DA, NE, HA, and ACh in the prefrontal cortex and hippocampus.

Figure 3A Possible regulation of several pro-cogntive neurotransmitters by cortical serotonin 1B heteroreceptors. A subpopulation of 5HT1B receptors may be localized directly upon presynaptic nerve terminals of NE, DA, ACh, and HA neurons. These are heteroreceptors because they are not located on 5HT neurons. These 5HT1B receptors are postsynaptic relative to their 5HT neurons, yet they are also presynaptic relative to NE, DA, ACh, and HA neurons. 5HT1B receptors are inhibitory and 5HT reduces the release of neurotransmitter from these neurons.

Figure 3B Possible disinhibition of several pro-cognitive neurotransmitters by vortioxetine acting at cortical serotonin 1B heteroreceptors. Vortioxetine is a partial agonist at 5HT1B heteroreceptors and possibly a functional antagonist. Thus, occupancy of cortical 5HT1B heteroreceptors localized on NE, ACh, DA, and HA neurons by vortioxetine would theoretically disinhibit these neurons and enhance the release of their neurotransmitters.
Clinical Implications
Abnormal connectivity of brain circuits is theorized to cause symptoms of psychiatric disorders such as depression, and psychotropic drugs hypothetically reduce these symptoms by changing this connectivity, thus improving the efficiency of information processing in specific brain circuits.Reference Insel, Cuthbert and Garvey 9 , Reference Stahl 10 Since there are numerous symptoms in major depression, including both emotional symptoms and cognitive symptoms, it is likely that there are numerous networks with altered connectivity involved in major depression.Reference Insel, Cuthbert and Garvey 9 , Reference Stahl 10 Psychopharmacologic agents such as vortioxetine that can change the release of many neurotransmitters and in more than one site (ie, multiple modes of action at multiple nodes within brain networks) theoretically have the possibility to change multiple symptoms linked to multiple circuits.Reference Insel, Cuthbert and Garvey 9 , Reference Stahl 10 Thus, it is possible that such actions of vortioxetine can explain not only its antidepressant actions,Reference Sanchez, Asin and Artigas 4 but also its unique procognitive actions in patients with major depression.Reference Katona, Hansen and Olsen 27 – Reference Mahableshwarkar, Zajecka, Jacobson, Chen and Keefe 29






