INTRODUCTION
Malaria is one of the major public health concerns in most of the tropical and sub-tropical countries. According to the WHO Malaria report for 2011, the global incidence of the disease was estimated at 216 million cases worldwide, of which 91% were due to Plasmodium falciparum. Malaria transmission is widely distributed in the Africa region (81%) followed by South East Asia (13%), and the Eastern Mediterranean region (5%) [1]. The WHO South East Asian Region comprises 11 countries, i.e. Bangladesh, Bhutan, Democratic People's Republic of Korea, India, Indonesia, Maldives, Myanmar, Nepal, Sri Lanka, Thailand and Timor-Leste where about 70% of the population are at risk of malaria and 26% at high risk. Maximum cases are being reported from India (61%), Indonesia (12%) and Myanmar (22%) which comprise 95% of cases and deaths, whereas the remaining 5% of malaria cases are from the other eight countries. About 2·15 million malaria cases with 1819 deaths were reported from this region in 2011. From 2000 to 2010, the incidence of malaria in the region was reduced from 30/1000 to 22/1000 population at risk, and malaria mortality rate was reduced from 4.2/100 000 to 3.0/100 000 population at risk [2].
In India, malaria has become a severe public health concern, 1·84 million cases and more than 1000 deaths are recorded annually [Reference Dhiman, Baruah and Singh3]. P. falciparum (51%) and P. vivax (49%) are the major malaria parasites distributed in India [4]. The transmission of malaria in most parts of the country is reported throughout the year due to ambient climatic conditions for growth and development of mosquitoes as well as parasites and also because of increasing ecological and human environmental changes [Reference Sharma5]. The most affected states are the Northeastern states, Odisha, Chhattisgarh, Madhya Pradesh, Jharkhand and West Bengal that account for nearly 60% of the total malaria cases. Of these states, the Northeastern states of India (Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura, Sikkim) contribute around 12–13% of total malaria cases, mostly due to the predominant parasitic infection of P. vivax and P. falciparum [Reference Mohapatra6–Reference Patra and Dev8]. Of all the Northeastern states of India, much of the research investigations related to malaria epidemiology and control are reported from Assam state [Reference Dev9]. According to the WHO malaria report for 2012, the state of Arunachal Pradesh is considered as highly endemic for malaria [2]. The districts of Changlang, Lohit, East Siang, Papum Pare, East Kameng, and West Kameng of Arunachal Pradesh are reported as high endemic districts for malaria [10]. The annual parasite incidence (API) rate in India has consistently fallen from 1·66/1000 in 2006 to 1·37/1000 in 2010. Similarly the API of Arunachal Pradesh has also decreased significantly from 37/1000 in 2006 to 18/1000 in 2010. However, there is sparse information available on malaria transmission dynamics in Arunachal Pradesh. This is the first detailed epidemiological study conducted in Arunachal Pradesh from 2006 to 2010.
Various types of mathematical models have been used widely for many infectious diseases [Reference Wu and Cowling11]. The Richards model is one such robust and logistic mathematical model for predicting various infectious diseases such as influenza H1N1 virus [Reference Hsieh, Fisman and Wu12], severe acute respiratory syndrome [Reference Hsieh and Cheng13], etc. This model generates an S-shaped curve and a turning point which illustrates the incidence of the pattern of disease. This data, allows the analysis of future disease incidence. We also used this model for the first time to predict malaria incidence patterns in Arunachal Pradesh in order to forecast the malaria scenario.
MATERIALS AND METHODS
Study area
Arunachal Pradesh is located in the Northeastern part of India situated between 26° 30′ N and 97° 30′ E. It represents only 1·18% of the total area of India and 3·34% of the total population of the country. The state is the most populous (population: 13·82 million according to the 2011 census survey) and geographically the largest state (83 743 km2) of the Northeast region, contributing significant numbers of malaria cases caused by both P. vivax and P. falciparum. The state is predominantly occupied by tribal populations living in low socioeconomic status conditions and in close association with forests. The state lies in the foothills of the Himalayas and is bounded by Bhutan to the West, China to the North-East, Myanmar (Burma) to the East and the plains of Assam to the South.
Collection of data
Arunachal Pradesh state comprises 15 districts with 91 primary health centres (PHCs) and 28 community health centres (CHCs) equipped with the facility for diagnosis and treatment of malaria. Malaria epidemiology data for the years 2006–2010 was collected from all PHCs and CHCs of Arunachal Pradesh and is used in this study.
Ethics statement
The study received ethical clearance from the Ethical Committee which was constituted in our institute (CSIR – Indian Institute of Chemical Technology, Hyderabad), affiliated to the Ministry of Science and Technology, Government of India. This Ethical Committee received approval to perform this research work. We declare that the data on epidemiology in this study was collected from the Directorate of Health Services, Government of Arunachal Pradesh and was analysed anonymously. The institutional review board waived the need for written informed consent from the participants as we were not directly involved in the epidemiology study. The information collected on this aspect of the study was carried out by a co-author and his group from the Directorate of Health Services, Government of Arunachal Pradesh. On no occasion were the names of the participants or identities collected, only the epidemiological data was made use of. The Directorate of Health Services, Government of Arunachal Pradesh is a statutory body authorized to perform this type of study.
Data analysis
The mean occurrence of P. vivax and P. falciparum malaria cases were compared between dry and wet seasons of a year in Arunachal Pradesh using the t test (SPSS v. 15·0 software; SPSS Inc., USA) and the level of significance was considered at 0·05. The slide positive rate (SPR) refers to the number of malaria-positive blood smears/total number of smears examined; the annual P. vivax index (AVI) = P. vivax cases/total population × 1000; the annual P. falciparum index (AFI) = P. falciparum cases/total population × 1000; and the annual parasitic index (API) = malaria cases/total population × 1000.
The Richards model
The Richards model is useful for capturing the temporal variations of disease prevalence, in particular the turning points (or peaks and valleys of the incidence S-shaped curve). By this curve it is easy to monitor the peak incidence for a particular wave of cases. The Richards model considers only the cumulative infected population curve with saturation in growth as the outbreak progresses, which is possibly caused by factors such as depletion of susceptibility in the population or increase in the vector density, and also the parasitic load in the community [Reference Hsieh and Cheng13]. It illustrates the usefulness of near real-time modelling in extracting valuable information regarding the outbreak directly from epidemic curves.
This model is derived from the logistic model which was proposed by Verhulst to model population growth and the equation is as follows:
where I′(t) is the population size at time t, r is the intrinsic growth rate and K is the carrying capacity. In 1959, Richards proposed the following modification of the logistic model to model the growth of biological populations:
where the prime (′) denotes the rate of change with respect to time. The parameter a provides a measure of flexibility in the curvature of the S shape exhibited by the resulting solution curve. As a model for the growth of an epidemic outbreak, C(t) is the cumulative number of infected cases at time t in days, K is the carrying capacity or total case numbers, r is the per capita growth rate of the infected population and α is the exponent of deviation from the standard logistic curve.
Application of the Richards model to predict malaria incidence in Arunachal Pradesh
The Richards model is mainly used to predict the spread of disease especially for epidemics [Reference Hsieh and Cheng13]. The role and efficacy of this model in characterizing endemic situations is relatively less explored as is the importance of the parameters in determining the trend of the disease. The Richards function is an increasing function on the whole real line that is bounded from below and above [Reference Hsieh and Cheng13]. The state of Arunachal Pradesh was taken as a case study and the monthly malaria incidence cases caused by P. vivax were taken for five successive years from 2006 to 2010. The cumulative data was used to fit the Richards model to estimate the total case numbers every year and also to estimate the growth rate of P. vivax cases each year. The monthly case data is taken annually and the cumulative infected case count for 12 time points (months of the year) were estimated for each year. The incidence curve for the cumulative infected cases for each year was plotted and it consisted of a single peak of high incidence resulting in an S-shaped curve, which is a characteristic of the Richards model. The malaria data fit to the Richards model was performed using Matlab software (www.mathworks.in) with the least squares approximation tool.
RESULTS
Malaria prevalence in Arunachal Pradesh
From the results it was inferred that malaria cases decreased significantly from 2006 to 2010, but the distribution of malaria prevalence varied greatly in the districts of Arunachal Pradesh. The API was found to be reduced from 37/1000 persons in 2006 to 18/1000 persons in 2010 (Fig. 1), but a high API (>61) was reported in the districts of East Siang, and Lower and Upper Dibang Valley (Fig. 2). Similarly, the SPRs of malaria gradually decreased from 14% in 2006 to 10% in 2010. The decrease in SPR is due to administration of chloroquine for the treatment of P. vivax and artemisinin-based combination therapy (artesunate + sulfadoxine pyrimethamine) for treatment of P. falcipuram cases. In the study period, two parasite species were identified, i.e. P. falciparum and P. vivax. Most of the cases were due to infection by P. vivax which was the predominant species with a prevalence of 72·1% followed by P. falciparum (27·9%). While estimating the number of malaria cases, infection due to P. vivax was found to have gradually decreased from 2006 (AVI 26%) to 2010 (AVI 13%) and the AFI was found to have decreased from 11% in 2006 to 5% in 2010. The AVI and AFI (per 1000 persons) of Arunachal Pradesh state, by district, are given in Table 1.
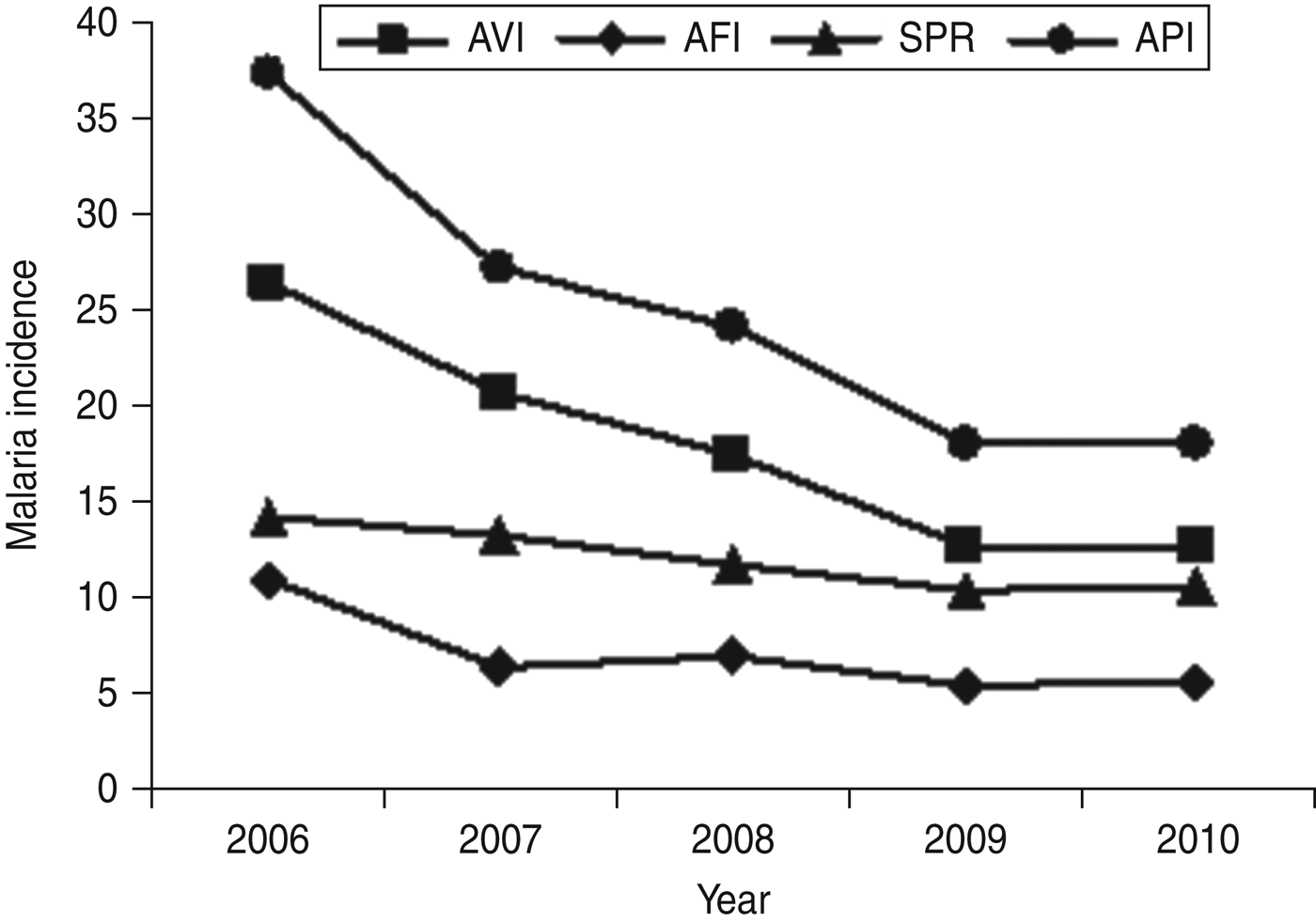
Fig. 1. Annual Plasmodium vivax incidence (AVI/1000 population), annual P. falciparum incidence (AFI/1000 population), smear-positive rate (SPR) and annual parasite incidence (API) in Arunachal Pradesh from 2006 to 2010.

Fig. 2. Spatial distribution of malaria incidence rates of the endemic districts of Arunachal Pradesh, India from 2006 to 2010. API, Annual parasite incidence.
Table 1. Annual Plasmodium vivax incidence (AVI) and annual P. falciparum incidence (AFI) for Arunachal Pradesh, by district and year, from 2006 to 2010

Seasonal trend of malaria cases in Arunachal Pradesh, 2006–2010
There was an apparent fluctuation in malarial trend observed in Arunachal Pradesh and monthly data reveal that malaria cases occurred throughout the year. Generally, malaria cases begin increasing in April and peak during the monsoon period, i.e. June and July, then gradually decline from August onwards (Fig. 3). The malaria data reveal that during the post-monsoon period the number of Plasmodium parasites gradually increased from September and peaked in October. The incidence of malaria then showed a sharp decline in November and December for the years 2006, 2009 and 2010 (Fig. 3). To obtain information on the incidence of malaria trend in comparison to the climatic seasons, the whole dataset was classified into dry (February–May) and wet (June–January) seasons; it was found that a higher number of malaria cases were observed mostly during the wet season (P < 0·003) compared to the dry season (Table 2). At the parasite species level, the maximum number of malaria cases was caused by P. vivax in the wet season, followed by P. falciparum. To understand the temporal distribution of malaria incidence between the dry and wet seasons for all the districts of Arunachal Pradesh, an increase in malaria cases was observed mostly during the wet compared to the dry season (Table 3).

Fig. 3. Monthly distribution of malaria cases in Arunachal Pradesh, India.
Table 2. Comparison of Plasmodium vivax (PV) and Plasmodium falciparum (PF) malaria cases from different season in Arunachal Pradesh during 2006–2010
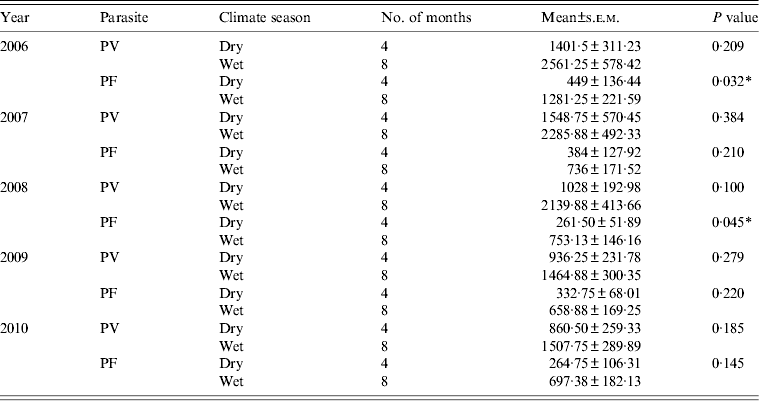
* P < 0·05.
Table 3. Correlation of malaria cases in dry and wet seasons in different districts of Arunachal Pradesh
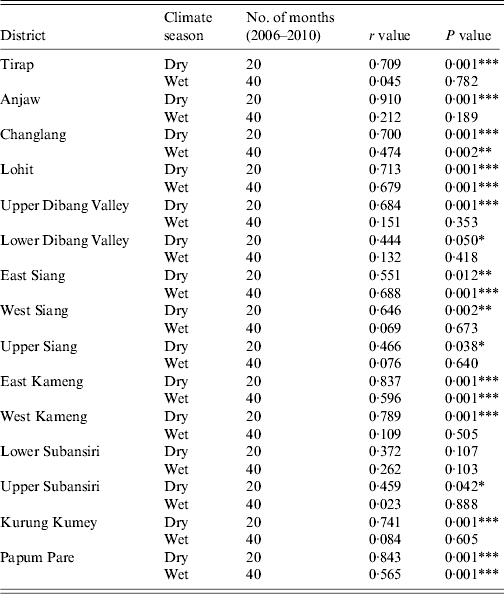
* P < 0·05, ** P < 0·01, *** P < 0·001.
Prediction model
Monthly malaria case data from 2006 to 2010 was used in the Richards model. The estimated parameters were obtained for all five years and the resulting theoretical curves were plotted and compared with the incidence curves of the real cumulative data (Fig. 4). The red line in each of the panels corresponds to the theoretical model curve and the blue line corresponds to the real data incidence curves. From this model the best-fit model for the years were 2006–2008 and 2010 with an error rate of 0·35%, 0·247%, 0·19% and 0·63%, respectively; for 2009 the error rate was 5·02% (Table 4). Figure 4 shows the predicted number of malaria cases, being high in February and reaching its peak between July and September for both the real and predicted curves. In Table 4, the parameter estimate K gives the predicted maximum number of infected cases in a particular year and it can be seen that the maximum number of infected cases was high for 2006 but the number decreased in the subsequent years. The parameter r represents the growth rate of the infection in each year and it can be seen that the maximum growth rate (r = 1·043) was observed in 2009 and for other years it was almost identical. α represents the exponent of deviation from the standard logistic curve.

Fig. 4 [colour online]. Model fit of cumulative malaria cases from 2006 to 2010 in Arunachal Pradesh, India.
Table 4. Actual and predicted malaria cases by the Richards model in the endemic state of Arunachal Pradesh, India from 2006 to 2010

DISCUSSION
The physical geography of Arunachal Pradesh is very distinct from the rest of the country but quite similar to the eco-climatic conditions of countries like Myanmar and Thailand [Reference Mohapatra6] where high numbers of malarial cases have been reported, mainly due to P. vivax followed by P. falciparum [Reference Dutta14]. Earlier reports suggest that the malaria pattern in Arunachal Pradesh is perennial and seasonally regulated. High transmission of malaria occurs during the monsoon and low transmission in the post-monsoon period [Reference Mohapatra6, Reference Dev, Hira and Rajkhowa15]. Persistence and outbreaks of malaria in this state may be due to poor inter-country coordinated vector control interventions, less awareness, difficult terrain and lack of healthcare services, low socioeconomic status and ideal climatic conditions for mosquito breeding and transmission of parasites [Reference Dash16]. To understand the pattern of disease transmission and the reason for high numbers of malaria cases, a detailed epidemiological study was conducted on malaria in Arunachal Pradesh from 2006 to 2010. The malaria epidemiological survey provides the baseline parasitological information of the population living in endemic districts of Arunachal Pradesh.
The variation in climate influences the prevalence of malaria and its survival is also dependent upon season, i.e. wet or dry seasons. While comparing malarial cases between dry and wet seasons, it was found that significantly higher numbers of cases were observed in the wet season than in the dry season of a year (P < 0·001). The data also analysed the number of mean cases of P. vivax and P. falciparum during dry and wet seasons for the study period; no significant correlation between number of P. vivax (P = 0·074) and P. falciparum (P = 0·140) cases was found. The predominance of malaria cases during the wet season could be attributed to favourable temperature and relative humidity that are essential for development of the parasite in the vector host (14·5–16·5°C for P. vivax and 16·5–18°C for P. falciparum) [Reference Martens17]. Similarly, seasonality of malaria was also reported in Mozambique [Reference Abellana18]. During the beginning of the wet season, increased numbers of breeding sites as well as suitable temperature (20–30°C) and relative humidity (>60%) are favourable for Anopheles to survive long enough to acquire and transmit the parasite effectively [Reference McMichael and Martens19]. It should be noted that local agricultural practices during the wet season, when mosquitoes find many sites for breeding, may be one of the factors for high transmission of malaria. During the latter part of the year the wet season experiences high temperatures causing the drying up of breeding sites, which results in fewer numbers of mosquitoes and low malarial incidence. A similar trend of results in all districts of Arunachal Pradesh was also observed in this study. Higher numbers of cases were reported in the wet season than in the dry season (Table 3). Malaria cases are declining steadily, which could be due to effective implementation of vector control interventions during onset of malaria cases, e.g. indoor residual spraying of dichlorodiphenyltrichloroethane (DDT; 50% WP) and use of impregnated bed nets by both state and central governments in endemic areas. The first round of DDT spraying is usually conducted during April–June, and the second round from July to August in each year before the onset of malarial cases [Reference Dev, Bhattacharyya and Talukdar20].
This study provides an example of using the Richards model to forecast malaria cases which will help in effective malaria control. The Richards model permits estimation of key epidemiological parameters based on cumulative case counts to study malaria incidence during 2006–2010. It should be noted that the model fits in most of the cases, as is evident from the curves. The accuracy of the model in predicting important parameters associated with the disease such as the maximum case numbers in each year and percentage of error is assessed Table 4.
In Table 4, the error percentages in the predicted maximum number of infected cases are fairly low for all years, except 2009, where the error is as high as 5%. Moreover, we observed that the other parameters, i.e. the growth rate r and exponential deviation α are nearly the same for all the years with the exception of 2009. This discrepancy in the 2009 predictions could perhaps be attributed to high incidence of malaria cases caused by P. vivax. However, the Richards model fits the data quite well for the remaining four years giving a fairly accurate estimation of the predicted maximum number of infected cases and even though there are deviations in the predicted results for 2009, the deviations are not large enough to dismiss the model. Hence, this Richards model can be applied to predict the number of infected cases in between the time-points already taken in each year and also to predict future cases [Reference Hsieh and Cheng13].
Given malaria incidence for any given time period, the procedure can be used to forecast the eventual severity of current phases in real time by estimating the carrying capacity K. One of the advantages of the Richards model for real-time modelling is that it offers a simple means of fitting a model to cumulative case counts, which smoothes out stochastic variations. Although there are numerous methodologies published, the Richards model can also be used to predict the maximum number of possible infected cases over a given period of time. Some competing methods require more extensive and detailed data than the Richards model, which requires only cumulative case data from an epidemic curve. As we have demonstrated here, the Richards model produces fairly stable and credible estimates of maximum case numbers, allowing these estimates to inform for evolving disease control strategies. In summary, we believe that the Richards model not only provides an important tool for rapid epidemic modelling in the face of a public health crisis, but can also be used to model endemic cases and give useful information about the disease progress in a population in any given time period.
ACKNOWLEDGEMENTS
The authors are grateful to the Director, CSIR – Indian Institute of Chemical Technology, for her encouragement and support. The authors also thank the Department of Science & Technology (DST), Government of India for sponsoring the project.
DECLARATION OF INTEREST
None.











