INTRODUCTION
Viral hepatitis is the 7th leading cause of mortality worldwide with hepatitis C virus (HCV) accounting for about half of this mortality [Reference Stanaway1]. Though HCV infection is a public health concern globally, the Middle East and North Africa (MENA) is the most affected region by this infection [Reference Stanaway1–3]. For 2015, MENA was estimated to have the highest incidence rate of all regions at 62·5 per 100 000 person-year, second largest incidence at 409 000 new infections per year, highest HCV antibody prevalence at 2·3%, and largest number of chronically infected people at 15 million [2].
Recent breakthroughs in HCV treatment, notably the introduction of highly effective direct-acting antivirals (DAA), have ushered a new era for controlling HCV and reducing its disease burden [Reference Flamm4]. Global targets have been set to eliminate HCV and reduce its mortality by 2030 [5, 6].
HCV is a blood borne pathogen transmitted parenterally such as through sharing of injections and use of contaminated medical equipment [Reference Prati7]. Patients undergoing hemodialysis (HD) are at a higher risk of HCV exposure due to sharing of dialysis machines [Reference Sy and Jamal8]. It has been estimated that HD increases the odds of acquiring HCV by five folds [Reference Sun9]. Characterizing HCV infection levels in HD patients and controlling its transmission through this mode of exposure are integral to improving the quality and healthcare utilization of HD. More specifically, this would lead to the prevention of unnecessary health complications such as liver disease and hepatic malignancies, and to a reduction in associated healthcare costs [Reference Bikbov10–Reference Fabrizi, Poordad and Martin12].
Against this background, we aimed to characterize HCV epidemiology among HD patients in the MENA region by: (1) systematically describing the evidence on HCV antibody incidence and prevalence in this population; (2) estimating the mean country-specific HCV prevalence in HD patients; (3) estimating HCV viremic rate in HD patients, that is the prevalence of HCV chronic infection (HCV RNA positivity) among antibody positive patients; (4) assessing associations with HCV prevalence in this population; and (5) assessing the frequency, distribution, and diversity of HCV genotypes in HD patients. This study was conducted under the umbrella of the MENA HCV Epidemiology Synthesis Project, an on-going effort to characterize HCV epidemiology and inform key public health research, policy, and programming priorities in MENA [Reference Mohamoud13–Reference Chaabna, Chemaitelly, Mumtaz and Abu-Raddad19].
METHODOLOGY
Data source
Our source of data was the MENA HCV Epidemiology Synthesis Project database. This database consists of several sub-databases that include an HCV antibody incidence database comprising 47 incidence studies among 29 600 participants, an HCV antibody prevalence database comprising 2543 antibody prevalence studies among 52 598 736 participants, an HCV RNA prevalence (among antibody positive persons) database comprising 178 RNA prevalence studies among 19 593 HCV antibody-positive participants, and an HCV genotype frequency database comprising 338 HCV genotype studies among 82 257 participants.
The MENA HCV Epidemiology Synthesis Project database was compiled through systematic searches of the literature [Reference Mohamoud13, Reference Fadlalla15–Reference Chemaitelly, Chaabna and Abu-Raddad17, Reference Chemaitelly20–Reference Al-Kanaani and Abu-Raddad22] informed by the Cochrane Collaboration handbook [Reference Higgins and Green23] and reporting the findings using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [Reference Moher24]. The searched literature included international databases (PubMed and Embase), regional databases, national databases, and the MENA HIV/AIDS Epidemiology Synthesis Project database [Reference Abu-Raddad25, Reference Abu-Raddad26], in addition to gray literature comprised of public health reports and routine data reporting, which are available from the authors upon request. The systematic reviews used broad search criteria with no language or year restrictions, to capture all publications pertinent to HCV since the discovery of the virus in 1989 [Reference Choo27, Reference Kuo28]. The systematic searches screened for duplicate studies and excluded them to avoid double counting of any single study.
The definition of the MENA region in these searches and in the present article included the 24 countries of Afghanistan, Algeria, Bahrain, Djibouti, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Mauritania, Morocco, Oman, Pakistan, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, the United Arab Emirates (UAE), and Yemen.
For the purpose of the present study, we also searched the literature (non-systematically) for studies of the population proportion of HD patients in MENA countries – that is the proportion of HD patients among the whole population in a given country. For estimating the number of people undergoing dialysis, the total population size in each country was obtained from the United Nations World Population Prospects database [29]. The MENA region estimate was calculated as a weighted (by population size) mean of available country measures.
Quantitative analyses
Meta-analyses
We conducted meta-analyses to estimate the country-specific mean HCV antibody prevalence. We further stratified HCV prevalence measures by year of publication and conducted meta-analyses for three different but consecutive temporal durations to descriptively examine changes in prevalence with time. Studies consisting of a minimum of 20 participants were included. We also conducted meta-analyses to estimate the country-specific mean viremic rate. Studies consisting of a minimum of 10 antibody-positive participants were included. In the event that the study reported HCV prevalence by different strata, such as age and sex, among others, the total sample size was replaced with stratified measures whenever the sample size requirement was fulfilled for each stratum.
Meta-analyses were conducted whenever we had three or more measures to be pooled using a DerSimonian–Laird random-effects model with inverse variance weighting [Reference Borenstein30]. The variance was stabilized using the Freeman–Tukey type arcsine square-root transformation [Reference Freeman and Tukey31]. Cochran's Q test was implemented to assess evidence for heterogeneity in effect size; a P-value < 0·1 was considered significant [Reference Borenstein32, Reference Higgin33]. The I 2 was calculated to assess the proportion of between-study variation in effect size (HCV prevalence or HCV viremic rate) that is due to actual differences in effect size between studies [Reference Borenstein32]. The prediction interval was calculated to assess the distribution of true effects around the estimated mean [Reference Borenstein32, Reference Higgins, Thompson and Spiegelhalter34].
Meta-regressions
We conducted meta-regressions on HCV prevalence studies to assess associations with HCV prevalence. Four types of potential predictors were specified a priori and included in the analyses: region within MENA, country income group, year of data collection, and sample size. Factors with a P-value < 0·1 in univariable analyses were eligible for inclusion in the final multivariable model. Factors with a P-value < 0·05 in the multivariable model were considered as significant predictors.
The region variable consisted of seven strata based on geographic proximity: Fertile Crescent (Iraq, Jordan, Lebanon, Palestine, and Syria); Gulf (Kuwait, Oman, Saudi Arabia, UAE, and Qatar); Horn of Africa (Yemen and Sudan); Maghreb (Algeria, Libya, Morocco, and Tunisia); Egypt; Iran; and Pakistan. This classification covers all MENA countries with available HCV data in HD patients.
The income group variable stratified countries according to their income group per World Bank classification [35]. Low middle-income countries included Egypt, Morocco, Pakistan, Sudan, Syria, Tunisia, and Yemen. Upper middle-income countries included Algeria, Iran, Iraq, Jordan, Lebanon, Libya, and Palestine. High-income countries included Kuwait, Oman, Saudi Arabia, Qatar, and UAE.
For the year-of-data-collection variable, we imputed the missing observations using the median of the observed values calculated by subtracting the year of data collection from the year of publication for each study. A sensitivity analysis was conducted with missing values for the non-imputed observations. The results were similar with no impact on statistical significance (Supplementary Table S1 on the Cambridge Journals Online website).
Genotype diversity
We analyzed the regional and country-specific frequency, distribution, and diversity of HCV genotypes among HD patients. The regional analysis was conducted based on actual frequency from available studies, and also as a weighted estimate by each country's population size. The frequency for each genotype was calculated with individuals testing positive for multiple genotypes contributing separately to the sum of cases for each genotype, as per earlier methodology [Reference Messina36]. Genotype diversity was assessed using Shannon Diversity Index [Reference Shannon37].
Statistical analyses were conducted using R studio version 3.3.2 [38] and StataSE version 13 [39].
RESULTS
HD in MENA
The population proportion of patients undergoing HD varied across countries (Table 1). The lowest reported was 250·2 per million population in Bahrain, and the highest reported was 665·4 per million population in Lebanon. Based on available population proportions, we estimated the country-specific number of patients undergoing HD (Table 1). The lowest was 327 patients in Bahrain, and the highest was 25 225 in Iran. The MENA region population proportion was 383·6 per million population yielding an estimate of 239 759 HD patients.
Table 1. Population proportion of hemodialysis (HD) in the Middle East and North Africa (MENA) [40]

a The population proportion of HD for the MENA region was estimated as a weighted mean of available country measures.
Scope of evidence
Out of the MENA HCV Epidemiology Synthesis Project database, we identified 21 HCV incidence measures among a total of 8857 HD patients (Supplementary Table S2), 205 HCV antibody prevalence measures among a total of 92 341 HD patients (Table 2), 31 HCV RNA prevalence measures among a total of 3172 HCV antibody-positive HD patients (Supplementary Fig. S1), and 31 HCV genotype frequency measures among a total of 2093 HCV RNA-positive HD patients (Table 6; Supplementary Fig. S2).
Table 2. Studies reporting hepatitis C virus (HCV) antibody prevalence among hemodialysis patients across the Middle East and North Africa

HD, Hemodialysis; Prev., Prevalence.
Out of the 24 included countries, data were available from 19 countries. The number of data points varied by country. Iran, Saudi Arabia, Tunisia, and Egypt contributed the largest number of data points. Several countries contributed as little as one data point.
HCV antibody incidence among HD patients
Egypt (n = 6), Lebanon (n = 6), and Morocco (n = 4) are the countries with the largest number of studies reporting HCV incidence among HD patients (Supplementary Table S2). Most studies had a follow-up duration ranging from 6 to 36 months, and reported incidence either as a risk of seroconversion or as an incidence rate. Seroconversion risk varied widely and was in the range of 0–100%. Incidence rate also varied substantially and was in the range of 0–14·7 per 1000 person-years. Incidence varied extensively even within the same country. For example, in Lebanon, HCV incidence rate varied from 0 to 14·7 per 1000 person-years across different geographical sites [Reference Rached120].
HCV antibody prevalence among HD patients
Iran (n = 41), Saudi Arabia (n = 39), and Egypt (n = 26) are the countries with the largest number of studies reporting HCV prevalence among HD patients (Table 2). HCV antibody prevalence varied widely within and across countries and was in the range of 0–100% with a median of 26·5%. For example, in Egypt, HCV prevalence varied from 10·0% to 100% across different geographical sites at different times.
Table 3 shows the pooled mean estimate of HCV prevalence by country, by temporal duration, and for the region. The country-specific mean estimate ranged from 7·3% (95% CI: 3·7–11·7%) in Lebanon to 65·5% (95% CI: 56·5–74·1%) in Egypt. HCV prevalence was 51·6% (95% CI: 46·1–57·1%) in years of publication 1989–1998, and decreased to 27·8% (95% CI: 23·1–32·8%) in 1999–2008, and 18·8% (14·5–23·5%) in 2009–2016.
Table 3. Pooled mean estimate for hepatitis C virus (HCV) antibody prevalence among hemodialysis patients across countries of the Middle East and North Africa
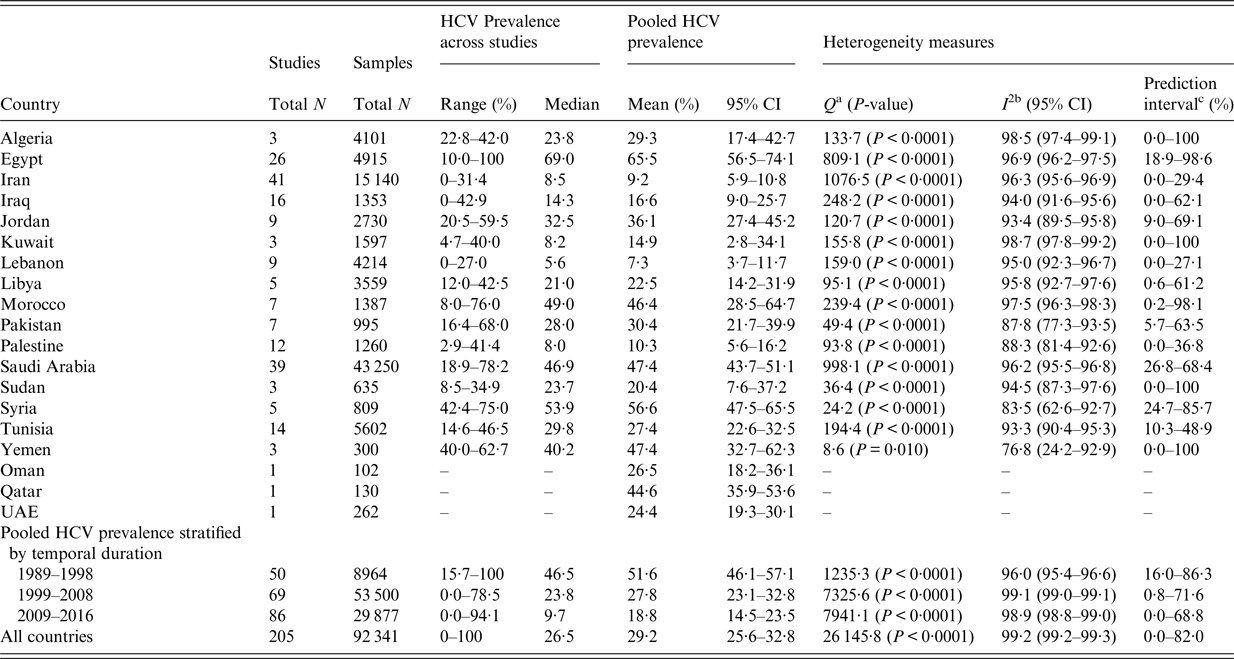
CI, Confidence interval; UAE, United Arab Emirates.
a Q: The Cochran's Q statistic is a measure assessing the existence of heterogeneity in effect size.
b I 2: A measure that assesses the magnitude of between-study variation that is due to differences in effect size across studies rather than chance.
c Prediction interval: A measure that estimates the 95% interval in which the true effect size in a new study will lie.
The mean estimate for the region was 29·2% (95% CI: 25·6–32·8%). Egypt, Syria, Saudi Arabia, Yemen, Morocco, and Qatar had a mean estimate exceeding 40%. There was strong evidence for heterogeneity in effect size (that is HCV prevalence) in all countries (P ⩽ 0·01). The vast majority of the variation was due to variation in effect size rather than chance (I 2 > 50%). The prediction intervals confirmed substantial variation in effect size in each country. Forest plots for the country-specific meta-analyses can be found in Supplementary Fig. S3.
HCV viremic rate among HD patients
Tunisia (n = 13) is the country with the largest number of studies reporting HCV viremic rate among HD patients (Table 4). For the rest of the countries, there were either few or no measures. HCV viremic rate varied across studies and was in the range of 19·1–93·3% with a median of 65·4%. The pooled mean estimate for HCV viremic rate across MENA was 63·0% (95% CI: 55·4–70·3%). There was evidence for heterogeneity in effect size estimates (here HCV viremic rate) across the region with a P < 0·0001. The I 2 for the pooled estimate was indicative of the variation being due to true differences in effect size rather than chance (I 2 = 94·0%). The prediction interval confirmed substantial variation in effect size. Forest plot for the regional meta-analysis can be found in Supplementary Fig. S1.
Table 4. Pooled mean estimate for hepatitis C virus (HCV) viremic rate among hemodialysis patients across countries of the Middle East and North Africa. HCV viremic rate is the prevalence of HCV chronic infection (HCV RNA positivity) among antibody-positive persons
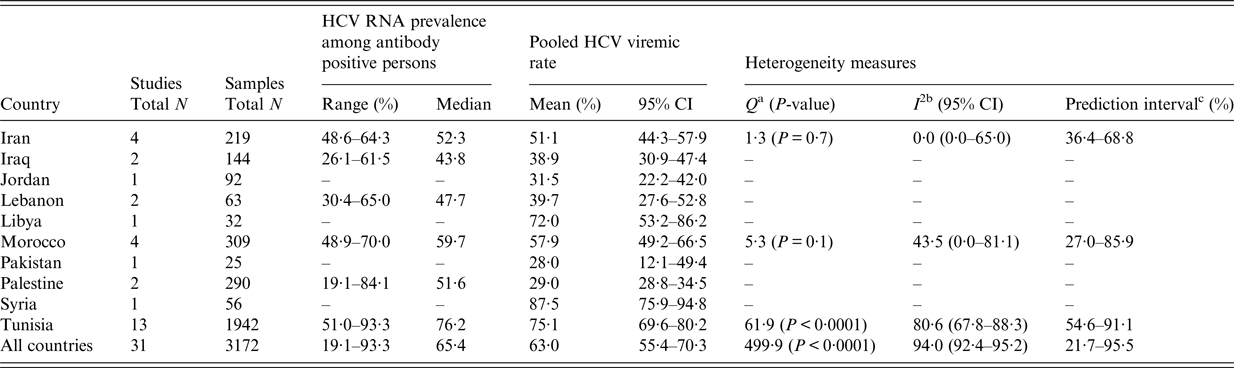
HCV, Hepatitis C virus; RNA, Ribonucleic acid.
a Q: The Cochran's Q statistic is a measure assessing the existence of heterogeneity in effect size.
b I 2: A measure that assesses the magnitude of between-study variation that is due to differences in effect size across studies rather than chance.
c Prediction interval: A measure that estimates the 95% interval in which the true effect size in a new study will lie.
Associations with HCV antibody prevalence among HD patients
Table 5 shows the results of the univariable and multivariable meta-regressions. Region, income group, and year of data collection were associated with HCV antibody prevalence in the univariable analysis (P-value < 0·1 for all three variables). Sample size was not associated with HCV prevalence (P-value > 0·7).
Table 5. Univariable and multivariable meta-regression models for hepatitis C virus (HCV) antibody prevalence among hemodialysis patients across the Middle East and North Africa
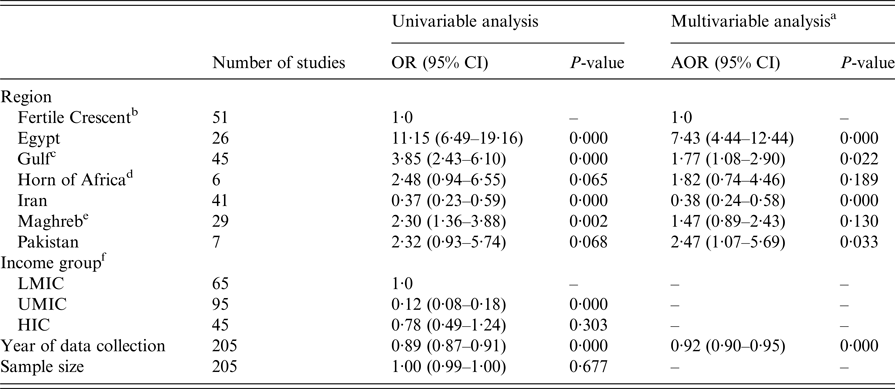
OR, Odds ratio; AOR, Adjusted odds ratio; LMIC, Low middle-income country; UMCI, Upper middle-income country; HIC, High-income country.
a The adjusted R-square for the full model was 54·48%.
b Fertile Crescent includes: Iraq, Jordan, Lebanon, Palestine, and Syria.
c Gulf includes: Kuwait, Oman, Qatar, Saudi Arabia, and Unite Arab Emirates.
d Horn of Africa includes: Yemen and Sudan.
e Maghreb includes: Algeria, Libya, Morocco, and Tunisia.
f Income group was removed from the multivariable analysis because of collinearity.
In the final multivariable analysis, region and year of data collection were included, but income group was not due to collinearity. The model explained 54·48% of the variation. Relative to the Fertile Crescent, the odds (for higher HCV prevalence) was much larger in Egypt; adjusted odds ratio (AOR) of 7·43 (95% CI: 4·44–12·44). It was also higher in Pakistan (AOR = 2·47; 95% CI: 1·07–5·69) and the Gulf region (AOR = 1·77; 95% CI: 1·08–2·90), but lower in Iran (AOR = 0·38; 95% CI: 0·24–0·58). Importantly, the AOR for the year of data collection was 0·92 (95% CI: 0·90–0·95) indicating declining HCV prevalence in HD patients year by year.
Risk factors for HCV infection among HD patients
Few studies assessed risk factors for HCV infection among HD patients. Duration and frequency of dialysis and exposure to blood transfusions were the most commonly reported risk factors [Reference Abdel-Wahab45, Reference Gohar51, Reference Goher52, Reference Saddik and El62, Reference Ramzi109, Reference Othman and Monem182].
HCV genotype and subtype distribution among HD patients
Table 6 shows the frequency, distribution, and Shannon Diversity Index of HCV genotypes among HD patients. Supplementary Fig. S2 also shows the distribution of HCV genotypes and subtypes.
Table 6. Frequency, distribution, and Shannon Diversity Index of hepatitis C virus (HCV) genotypes among hemodialysis patients across the Middle East and North Africa
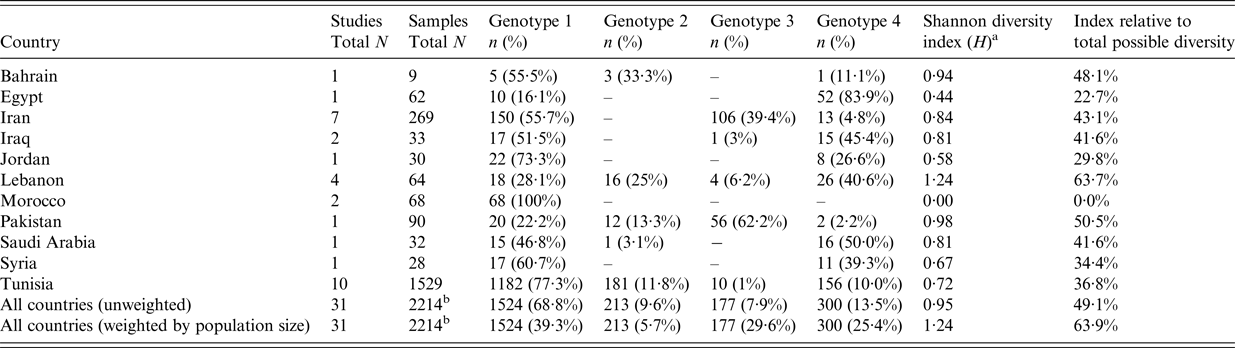
No data were found for HCV genotypes 5, 6, and 7.
a The maximum value for Shannon Diversity Index is 1·95 assuming full genotype diversity of seven HCV genotypes [Reference Chemaitelly, Chaabna and Abu-Raddad17, Reference Shannon37].
b Each individual testing positive for multiple genotypes contributed separately to the sum of cases for each genotype.
The vast majority (92·3%) of viremic HD patients were infected by a single strain (Supplementary Fig. S2). At the regional level, four HCV genotypes were documented in HD patients: genotype 1 (68·8%), genotype 2 (9·6%), genotype 3 (7·9%), and genotype 4 (13·5%). Weighted by country population size, the regional genotype distribution was: genotype 1 (39·3%), genotype 2 (5·7%), genotype 3 (29·6%), and genotype 4 (25·4%). No cases of genotypes 5, 6, and 7 were reported.
Genotype 1 was commonly found in Morocco (100%), Tunisia (77·3%), Jordan (73·3%), Syria (60·7%), Iran (55·7%), Bahrain (55·5%), Iraq (51·5%), and Saudi Arabia (46·8%). It was also common in Lebanon (28·1%), Pakistan (22·2%), and Egypt (16·1%).
Genotype 4 was commonly found in Egypt (83·9%), Saudi Arabia (50·0%), Iraq (45·4%), and Lebanon (40·6%). It was also present in the rest of the countries apart from Morocco. Pakistan was the only country where genotype 3 was the most common genotype (62·2%). Genotype 3 was also common in Iran (39·4%), but otherwise rare in the rest of the countries. Genotype 2 was common in Bahrain (33·3%) and Lebanon (25·0%), and somewhat common in Pakistan (13·3%) and Tunisia (11·8%), but otherwise rare.
For genotype 1, subtype 1a and 1b were commonly observed in MENA (Supplementary Fig. S2). Subtypes 2a, 2b, 2c, 3a, 3b, and 4a were also detected in this region. Combinations of the above genotypes and subtypes were observed among multiply infected individuals.
Genotype diversity varied across countries. It was highest in Lebanon with a relative Shannon Diversity Index of 63·7% (score: 1·24 out of a maximum of 1·95) and lowest in Morocco 0·0% (score: 0·0 out of a maximum of 1·95). For the region as a whole, the relative Shannon Diversity Index was 49·1% (score: 0·95 out of a maximum of 1·95) for the unweighted analysis, and 63·9% (score: 1·24 out of a maximum of 1·95) for the weighted analysis by country population size.
DISCUSSION
Through a comprehensive investigation of HCV epidemiology among HD patients in MENA, we found that there is ongoing and considerably high HCV incidence in this population across the region. However, the incidence rate varied between countries and across different settings within the same country (Supplementary Table S2). We also found high HCV prevalence in HD patients, with the prevalence varying substantially across the region and within each country. About one-third (29·2%) of HD patients have already been infected with HCV (Table 3), with two-thirds of them (63·0%) being chronic carriers (Table 4) that can potentially transmit the infection to other patients through dialysis. We also found substantial diversity of HCV genotypes in HD patients, with genotype 1 being the most common at the regional level (Table 6). Importantly, we found that HCV prevalence in HD patients is on a declining trend (Tables 3 and 5).
These findings suggest that the standard of infection control in dialysis differs across countries and across dialysis units within each country. They also indicate that extensive improvement is needed to control HCV transmission among HD patients. Fortunately, improvements appear to be already taking place as validated by the declining trend in prevalence. These findings highlight further the importance of addressing HCV infection and disease burden in HD patients, especially considering the recent availability and increasing affordability of DAAs for HCV treatment [Reference Flamm4], and that the number of patients undergoing dialysis is rising rapidly with the aging of the population and growing prevalence of chronic diseases that lead to renal disease [40, 195–Reference Anand, Bitton and Gaziano197].
Our pooled mean estimate for HCV prevalence indicated that HCV prevalence among HD patients in MENA is higher than that in other regions such as Europe (7·2%) [Reference Zampieron198], the USA (7·9%) [Reference Patel199, Reference Finelli200], and the Asia-Pacific region (range across countries of 1–18%) [Reference Johnson201]. This finding may not only suggest inferior standards of dialysis in MENA, but may also reflect the higher background HCV prevalence in the whole population in this region [2, 3]. Indeed, HCV prevalence among HD patients in MENA countries reflected in part HCV prevalence in the population at large in each country. For example, HCV prevalence among HD patients in Egypt and Pakistan was much higher than that in other MENA countries (Table 5), reflecting the higher prevalence in the wider population in these two countries [Reference Mohamoud13, Reference Al-Kanaani and Abu-Raddad22].
The high HCV incidence and prevalence in HD patients appear to reflect improper application of infection control measures, such as by healthcare personnel to maintain hands hygiene [Reference Patel199, 202], to change gloves routinely [Reference Patel199, 202], and to clean the dialysis unit and equipment properly between patients [Reference Sy and Jamal8, Reference Zampieron203]. With the continuing conflict emergencies in several countries and the large refugee and migrant populations, overloading dialysis units and depletion of medical resources is a growing concern [204, Reference Daw and Dau205]. These emergencies appear to have led to reuse and sharing of supplies and equipment intended for single usage such as infusion vials and machine filters [Reference Daw and Dau205, Reference Fabrizi and Messa206]. The lingering emergencies may undermine the recent improvements and reverse the trend of declining HCV prevalence in HD patients.
We found substantial diversity in the circulating HCV genotypes in HD patients across countries. However, this diversity appeared to reflect the distribution of circulating genotypes in each country [Reference Mahmud207]. For example, the dominant genotypes among HD patients in Egypt and Pakistan were genotypes 4 and 3, respectively (Table 6), similar to the dominant genotypes in the wider population in these two countries [Reference Mahmud207]. These findings may indicate overlapping transmission networks where HCV is circulating from one population to another through different modes of exposure including dialysis.
A review of HCV among HD patients in the Middle East has been recently published [Reference Ashkani-Esfahani, Alavian and Salehi-Marzijarani208]. Its findings agreed overall with our findings despite differences between the two studies in the focus, scope, and analysis plans. Our study covered more countries in MENA over a longer duration, examined analytically trends and associations, and reported a broader set of outcome measures and analyses (such as for the genotype distribution). In total, 205 studies were included in our analyses compared to 56 studies in Ashkani-Esfahani et al. study [Reference Ashkani-Esfahani, Alavian and Salehi-Marzijarani208]. Both studies concluded that there is high HCV prevalence in HD patients that needs to be addressed through targeted interventions.
Our study had several limitations. The availability of data varied from one county to another and we did not identify any data for five MENA countries (Afghanistan, Bahrain, Djibouti, Mauritania, and Somalia). Sample size varied also across studies and the sampled HD populations may have been sampled from specific geographic areas within a given country, and may not be representative of the wider HD population in the country. Despite these limitations, we were able to identify a large volume of data for MENA countries that allowed us to conduct different types of analyses, generate multiple inferences, and produce a comprehensive mapping of HCV epidemiology among HD patients in this region.
CONCLUSIONS
Our findings revealed ongoing HCV incidence and high HCV prevalence among HD patients in MENA, but incidence and prevalence appear to be declining year by year. About one-fifth of HD patients are chronic carriers of HCV infection, in need of HCV treatment, and potentially can transmit the infection to other HD patients. In context of rapidly growing HD patient population, these findings highlight the need to improve standards of infection control in dialysis in MENA.
Moreover, in context of recent availability and increasing affordability of DAAs for HCV treatment, these findings highlight the urgency to address HCV infection and disease burden in HD patients. Governments that are reluctant to take on national level HCV elimination projects, could initially focus on HD patients as a candidate population for micro-elimination as a way to advance the agenda for HCV DAA treatment. With HCV circulation among HD patients being a major mode of HCV transmission, tackling this infection and disease burden is critical to HCV global elimination and mortality reduction targets by 2030.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268817002242.
ACKNOWLEDGEMENTS
This publication was made possible by NPRP grant number 9-040-3-008 from the Qatar National Research Fund (a member of Qatar Foundation). The findings achieved herein are solely the responsibility of the authors. The authors are also grateful for infrastructure support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine-Qatar.
DECLARATION OF INTEREST
The authors have no competing interest to declare.









