INTRODUCTION
During investigations of epidemic frog mortality in Britain, a novel fatal systemic haemorrhagic disease of common toads (Bufo bufo) was discovered. This disease resembles a systemic haemorrhagic disease of common frogs (Rana temporaria) in Britain, which is one of a range of fatal disease syndromes, characterized by systemic haemorrhages, skin ulceration or a combination of these lesions, caused by ranavirus infection [Reference Cunningham1, Reference Cunningham2]. All three syndromes were reproduced in the laboratory following exposure to ranavirus, family Iridoviridae, isolated from diseased frogs found dead in the wild and Koch's postulates were fulfilled through the re-isolation of virus from experimentally induced lesions [Reference Cunningham2]. No ulcerative skin disease has been found in toads in Britain.
Ranavirus was isolated from naturally diseased toads [Reference Cunningham3]. Two of these isolates (BUK2 and BUK4) were characterized using restriction fragment length polymorphism (RFLP) profiles, SDS–PAGE protein profiles and sequencing of parts of the virus genome and were found to be similar, but not identical, to ranavirus from common frogs [Reference Cunningham3, Reference Hyatt4].
In order to evaluate if ranavirus isolated from common toads with systemic haemorrhages could infect and cause disease in common frogs, we repeated the frog ranavirus transmission studies conducted by Cunningham et al. [Reference Cunningham2] using ranavirus isolated from a naturally diseased common toad.
METHODS
Frogs
Adult frogs were wild-caught in County Cavan, Eire as no reports of epidemic disease in wild amphibians, or of frogs with lesions consistent with ranavirus infection, have been reported from Ireland. All animals captured were seronegative for ranavirus using a competitive antibody capture ELISA [Reference Zupanovic5], for which the antibodies had been raised against epizootic haematopoietic necrosis virus (EHNV) and had been shown to react against all known ranaviruses [Reference Hyatt4–Reference Hengstberger6]. The animals had been maintained in captivity for 11 months before use, throughout which they had remained healthy. The housing and husbandry of the frogs was as described by Cunningham et al. [Reference Cunningham2]. This experiment was conducted under a Home Office Project Licence and with the approval of the Zoological Society of London's Ethics Committee.
Virus
Ranavirus isolated from the kidney of a wild common toad (ZSL post-mortem reference 753/95) naturally diseased with systemic haemorrhages [Reference Cunningham3] (isolate no. 67) was used in this study. This toad was found dead at the same time and in the same location (Claygate, Esher, Surrey) as toad ref. 752/95, from the kidney of which the fully characterized ranavirus, BUK4, had been isolated [Reference Cunningham3, Reference Hyatt4]. Isolate no. 67 was cultured for three passages (P3) in fat-head minnow epithelial (FHM) cells [European Collection of Cell Cultures (ECACC) cell line no. 88102401], as described by Cunningham [Reference Cunningham3]. The harvested cell culture fluid contained a virus titre of 105·7 TCID50/ml. Isolate no. 67 was used because isolate BUK4 was unavailable to this study, as it was archived under biosecurity restrictions in the CSIRO Australian Animal Health Laboratory, Geelong, Australia.
Experimental exposure of frogs to toad ranavirus
Five common frogs were inoculated intraperitoneally (i.p.) and subcutaneously (s.c.) with a total of 0·5 ml (0·25 ml inoculated into each site) of harvested P3 no. 67 virus in cell culture fluid, following the method used for UK frog ranavirus transmission studies [Reference Cunningham2]. Five frogs were mock-infected by intraperitoneal and subcutaneous inoculation with a total of 0·5 ml (0·25 ml inoculated into each site) of tissue culture fluid harvested from an equivalent flask of FHM cells, but which had not been used to culture virus.
Throughout the study, great care (including temporal and spatial distance) was taken to avoid cross-contamination between frogs or containers. Following inoculation, each frog was inspected briefly on a daily basis and examined clinically every 3 days. Any animal found in distress was euthanized by immersion in a 0·4% aqueous solution of tricaine methane sulphonate (MS222, Thomson & Joseph Ltd, Norwich, UK) until anaesthetized, followed by stunning and pithing. All frogs exposed to virus were examined systematically post mortem. Two mock-infected frogs were killed and examined at the end of the experiment (30 days post-exposure). Post-mortem examinations were conducted immediately following euthanasia or within 12 h of death.
Ranavirus detection
Samples of kidney were taken from each frog necropsied, fixed in 2·5% glutaraldehyde or stored frozen, and examined for the presence of viruses using both transmission electron microscopy (TEM) and cell culture, as described by Cunningham [Reference Cunningham3].
Statistical analyses
Fisher's exact test for small sample sizes [Reference Kirkwood7] was used to examine for associations between exposure to virus and the development of disease and for associations between the development of disease and the presence of virus.
RESULTS
Transmission experiment
A summary of the results of this experiment is presented in the Table. All five of the frogs inoculated with virus died (or were euthanized) with systemic haemorrhages (Fig.) 6–8 days post-inoculation. No virus-inoculated frog developed skin ulceration. All five mock-infected frogs remained healthy. No lesions were found in the two mock-infected frogs necropsied at the end of the experiment.
Table. Outcomes following exposure of frogs to ranavirus (isolate no. 67) cultured from a naturally diseased toad
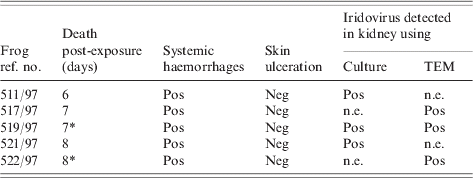
Neg, Negative; Pos, positive, n.e., not examined.
* Euthanized.

Fig. Frog ref. 522/97, which was euthanized with systemic haemorrhaging 8 days post-exposure, via i.p. and s.c. inoculation, to virus no. 67 (isolated from a toad which died of haemorrhagic syndrome-like illness). The carcase has been skinned to show extensive haemorrhaging (a) within the musculature of the dorsal left flank and (b) within the soft tissues of the hind legs, most notably of the hind feet.
Virology
Ranavirus was detected (via culture, TEM or both of these techniques) in the tissues examined from all of the virus-inoculated animals. Tissues from the mock-infected frogs examined were negative for ranavirus.
Statistical analyses
All five frogs exposed to cultured virus died with systemic haemorrhages whereas none of the five mock-infected animals became sick (Fisher's exact test=0·008). Ranavirus was detected in the tissues of all five frogs that developed disease, but from neither of the two mock-infected animals tested (Fisher's exact test=0·048). There was, therefore, a positive association both between exposure to toad ranavirus and the development of disease and between the development of disease and the presence of ranavirus.
DISCUSSION
A newly emergent epidemic disease of common frogs in Britain, characterized by systemic haemorrhages and skin ulceration, has previously been found to be caused by ranavirus infection [Reference Cunningham1–Reference Cunningham3]. During frog mortality field investigations, common toads were also found dead with systemic haemorrhagic disease [Reference Cunningham3]. In this study, the toad disease was successfully transmitted to frogs through the inoculation of ranavirus cultured from a naturally affected toad, demonstrating that the toad ranavirus is able to infect frogs. Moreover, these results indicate that, as is the case for the epidemic disease of frogs, the toad disease is caused by ranavirus infection.
In the field, outbreaks of haemorrhagic disease can affect both frogs and toads concurrently. Although this might be due to the coincidental occurrence of two similar, but species-specific, agents requiring the same combination of environmental, or other, conditions (e.g. temperature) to become pathogenic, the results of this study indicate that a more likely explanation is that the same virus causes haemorrhagic disease in both frogs and toads and that this virus is naturally transmissible between the two species. Furthermore, both the lesions produced and the time to death following i.p.+s.c. inoculation with virus no. 67 are similar to those produced by the i.p.+s.c. inoculation of frogs with isolates (RUK11 and RUK13) of ranavirus cultured from naturally diseased frogs [Reference Cunningham2], indicating that the same, or similar, viruses are affecting both frogs and toads in the field. This conclusion is supported by molecular studies on ranavirus isolates from British amphibians, in which toad isolates group with, or closely to, those from frogs according to physical properties, restriction endonuclease digestion profiles and phylogenetic comparisons of genome sequences [Reference Hyatt4].
Although the common frog is more notably affected by ranavirus disease in Britain than the common toad, this might be because of the higher visibility of frogs compared to toads. Frogs are gregarious animals which spend much of the year within and around ponds, while toads are solitary and gather at ponds only during the breeding season when the water and the average daily air temperatures are low (<10°C). The optimum temperature range for ranavirus growth in vitro is 15–30°C [Reference Cunningham3]. Ranavirus disease outbreaks usually occur in the mid to late summer months when many tens or hundreds of dead frogs can be found at a single site, whereas dead toads are infrequently found [Reference Cunningham3].
Prior to the investigations into epidemic frog mortality, ranaviruses were unknown in Britain. It has since been shown, using molecular studies, that ranaviruses infecting frogs and toads in Britain most likely result from a recent incursion from North America [Reference Cunningham3, Reference Hyatt4]. While these viruses appear to be highly pathogenic to the common frog and the common toad, the effect of ranavirus disease on British amphibian populations is unknown. Unexplained declines of common toad populations in south-east England, however, have been reported recently [Reference Carrier and Beebee8]. South-east England is the region of Britain which, each year, has had the highest incidence of ranavirus disease [Reference Cunningham3]. It is possible that ranavirus infection is a factor in these declines.
ACKNOWLEDGEMENTS
We thank Mike Lovett and Gill Bell for technical assistance and Tom Langton for support and advice. This work was funded by the Institute of Zoology, London.
DECLARATION OF INTEREST
None.




