INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) are a group of pathogenic E. coli strains capable of producing toxins which resemble those produced by Shigella dysenteriae [Reference O'Brien1]. Because of the numerous outbreaks and potential life-threatening complications (haemolytic uraemic syndrome; HUS), STEC are considered an emerging pathogen [Reference Pitout and Laupland2]. As required by the German Protection against Infection Act [3], data on STEC and HUS infections are collected systematically based on reports by laboratories and physicians. Germany registers on average about 1100 STEC cases/year (70 HUS cases/year), with most cases occurring in regions where cattle density is high, such as Bavaria and Lower Saxony [Reference Frank4]. However, in 2011, a serious outbreak of E. coli producing both Shiga toxin- and extended-spectrum β-lactamases (ESBL) caused 4907 STEC cases (18 deaths) and 880 HUS cases (32 deaths), mainly in Northern Germany [Reference Buchholz5].
STEC outbreak management in Germany includes screening close contacts of notified cases to detect further cases that might be asymptomatic. Any child testing positive for STEC needs approval of the local health authorities before they are allowed to return to nursery, kindergarten or school, and must comply with specified protection measures (German Protection against Infection Act §34/2) to prevent transmission to other children. A common measure used by local public health offices is to exclude asymptomatic children who are contacts of confirmed cases from nurseries until three consecutive stool samples test negative for STEC. This measure has substantial socioeconomic effects: STEC is shed for 20 days on average (up to 71 days) [Reference Wahl6, Reference Dabke7] and parents often need to take compassionate leave to care for their children at home. In addition, school-age children might fall behind in school.
Similarly, our knowledge about extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E) prevalence in asymptomatic children is limited. The number of asymptomatic adults colonized with ESBL-E in the community has also been increasing [Reference Kader, Kumar and Kamath8, Reference Valenza9]. Recent estimates of the proportion of ESBL-E carriers in asymptomatic adults vary between countries and range from 5·8% in Switzerland [Reference Geser10] to 65·7% in Thailand [Reference Luvsansharav11].
ESBL producers have been found across Enterobacteriaceae, including Klebsiella spp. and E. coli [Reference Kader, Kumar and Kamath8]. ESBL are capable of inactivating β-lactam antimicrobials and they are frequently plasmid encoded, which supports the spread of antimicrobial resistance [Reference Livermore and Woodford12]. Usage of β-lactam antibiotics (e.g. penicillin, first-, second-, and third-generation cephalosporins) increases the conferring of bacterial resistance [Reference Schechner13], and the prescription rate of antibiotics in German children aged <6 years is higher than for any other age group [14]. Initially, ESBL-E were only observed and screened in hospitals [Reference Coque, Baquero and Canton15], where they have caused nosocomial outbreaks in neonatal units [Reference Haller16]. The aim of our study was to estimate the prevalence of STEC and ESBL-E in asymptomatic children and identify factors associated with STEC and ESBL-E carriage to guide risk assessment and management.
MATERIAL AND METHODS
Study design and data collection
We performed an anonymous cross-sectional study between April and September 2014 in four districts in Lower Saxony in asymptomatic nursery children aged 0–6 years. Lower Saxony is a federal state in northern Germany, which has a population of about eight million and is rather rurally structured with average population density of about 168 inhabitants/km2. The districts were selected with regard to cattle density. We chose two districts with a high cattle density (range 1·3–1·77 livestock units/ha) and two with low density (range 0·21–0·44 livestock units/ha) [17]. Being asymptomatic was defined as absence of diarrhoea, i.e. if the question ‘suffering from diarrhoea within the previous 7 days’ in the questionnaire was answered in the negative.
In cooperation with the local public health authorities, we recruited nurseries by using convenience sampling. We provided participating nurseries with study packages containing information material (posters and leaflets in German), questionnaires and sample kits. Parents completed a questionnaire, signed a consent form and collected a stool specimen of their child. The data were linked by using a common survey participant identification number.
Questionnaire
A questionnaire was designed to collected demographic data (age, sex and district) as well as information about food consumed (including meat and milk products), contact with animals, outdoor activities (e.g. swimming), travelling, and symptoms 7 days preceding answering the questionnaire, and antibiotic treatment during the preceding 6 months. The questions were selected from other epidemiological studies focusing on children [Reference Ziehm18] and were a main part of the ethics committee evaluation. The questionnaire was checked for completeness and plausibility. The inter-questionnaire consistency was tested by comparing conflicting information between two questions. If the answers indicated that the child had suffered from diarrhoea within the previous 7 days, the child was excluded from the study.
Microbiology
The medical laboratory of the Governmental Institute of Public Health of Lower Saxony analysed the stool samples. The stool specimens were enriched in mTSB (tryptone soya broth containing mitomycin C; R-Biopharm AG, Germany) and incubated at 37 °C for 18–24 h. Nucleic acid isolation was performed using the DNA QIAamp Mini kit (Qiagen, Germany) with 200 μl elution volume. Afterwards, the specimens were examined for Shiga toxin (Stx)-producing E. coli and Shiga toxin genes (stx1 and stx2) using Shiga toxin-specific DNA sequences by polymerase chain reaction (PCR) [Reference Reischl19]. The sensitivities and specificities of the LC-PCR assays were each 100% for the stx1 gene and 96% and 100%, respectively, for the stx2 gene [Reference Reischl19]. For stx-positive tested isolates no follow-up tests (e.g. serotyping) or cultivation were performed.
Enterobacteriaceae producing ESBLs were cultured on selective chromogenic culture media (CHROMagar™ ESBL, MAST Group, Germany). Microorganisms were identified based on their metabolic profile, using an automated system (VITEK 2 compact; bioMérieux, France). No further ESBL confirmatory tests were performed.
Statistical analyses
Microsoft Access2003 (Microsoft Corp., USA) was used as the database system. Descriptive statistics were used to characterise carriers and non-carriers according to age, sex and district. The prevalence of carriage for both STEC and ESBL-E was calculated. To identify factors associated with carriage, we used χ 2 test and Fisher's exact test. Exposures with P < 0·05 were regarded as statistically significantly associated. The strength of the association was assessed with odds ratios (ORs) and 95% confidence intervals (CIs). We conducted the statistical analyses using Stata/SE12 software (StataCorp., USA). Due to the low numbers of positive specimens (see Results section), a stratified analysis was not performed.
Ethics approval
Ethics approval for the study was granted in March 2014 by the Ethics Commission of the State Chamber of Medicine in Lower Saxony (Landesärztekammer Niedersachsen, Hannover, Germany, BO/45/2013).
RESULTS
We recruited 59 nurseries, of which 30 were from districts with high cattle density and 29 were from districts with low cattle density. In total, 1420 study packages were distributed to parents and 225 stool specimens (15·8%) were returned to the Governmental Institute of Public Health of Lower Saxony together with consent forms and questionnaires. Two participants reported diarrhoea in the preceding 7 days and therefore the two stool specimens were subsequently excluded.
The median age of study participants was three years (interquartile range 3 years). Fifty-six percent (n = 124) were male and 44% lived in districts with high cattle density.
STEC were found in stool specimens of two children (one with the presence of stx1 and stx2 and one with stx1 only). The latter child reported diarrhoea during the last 7 days and was therefore excluded from the study. Hence, the prevalence of STEC-positive asymptomatic study participants was 0·5% (n = 1/224, 95% CI 0–1·3). Due to the low number of STEC carriers, we refrained from further statistical analyses.
Six specimens tested positive for ESBL-E: four samples contained ESBL-producing E. coli, one contained both E. coli and Citrobacter and one ESBL-producing Klebsiella. One child with ESBL-producing E. coli stated having had diarrhoea during the last 7 days and was excluded consequently from the study. The prevalence of ESBL-E-positive asymptomatic study participants was 2·3% (n = 5, 95% CI 0·3–4·2).
Univariable analyses revealed no significant difference in sex, age and district between ESBL-E carriers (n = 5) and non-carriers (n = 219). One of the ESBL-E carriers had been hospitalized during the previous 6 months. ESBL-E carriers reported a significantly higher use of antibiotics during the preceding 6 months than non-carriers (see Table 1). Children with ESBL-E positive stool specimens were 14 times more likely to have consumed raw milk (milk that has not been pasteurized or cooked), 13 times more likely to have received antibiotic treatment during the preceding 6 months and eight times more likely to have had contact with pet rodents. There were too few observations within some categories to perform a multivariable analysis.
Table 1. Univariable analysis of covariates for extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E)carriage in asymptomatic nursery children (n = 224) in Lower Saxony, 2014
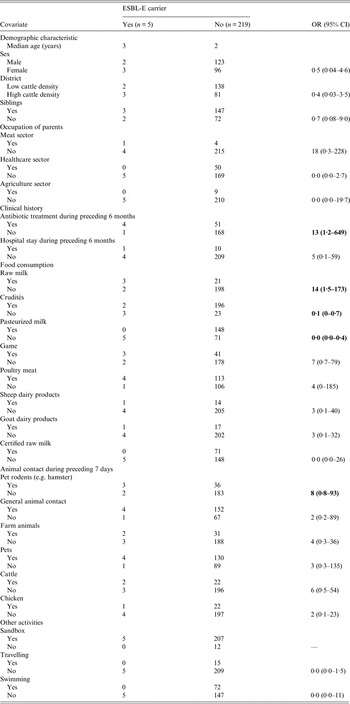
OR, Odds ratio; CI, confidence interval.
Statistically significant associations (P < 0·05) appear in bold.
Out of the 224 participants, 24 (11%) reported raw milk consumption and 17 (71%) of the 24 lived in districts with high cattle density.
DISCUSSION
In this cross-sectional study, we identified a low STEC prevalence and an average ESBL-E prevalence in asymptomatic nursery children aged 0–6 years in Lower Saxony. Univariable analysis revealed that raw milk consumption, contact with rodents and antibiotic treatment was more frequent in ESBL-E carriers.
We found a surprisingly high proportion of raw milk consumption in our total study population.
Prevalence of STEC
On some occasions, contact screening has revealed a high proportion of asymptomatic STEC carriers and typing has shown the presence of different strains. This indicates a high prevalence of asymptomatic carriage. Fairly high numbers of asymptomatic carriers have been found during investigation of local outbreaks [Reference Vogelsang20, Reference Dreesman, Röttgers and Mellmann21]. For example in 2012, contact screening in 31 farm employees in Lower Saxony identified nine asymptomatic carriers [22]. The virulence pattern of the pathogenic factors deviated widely. If this reflects a general high prevalence of asymptomatic carriage in the population, it would be inadequate to exclude a child who has been identified by chance during a contact investigation. The current policy for exclusion is based on the assumption that asymptomatic carriers are rare. However, the actual prevalence of asymptomatic STEC carriers in German children has not been assessed yet. Considering the current exclusion criteria from nurseries and schools, a known STEC prevalence in asymptomatic children would be informative for policy makers to decide on appropriate control measures.
The observed STEC prevalence of 0·5% in asymptomatic children was lower than expected and not consistent with the proportion of carriers reported in outbreak investigations (screening of contact persons) in Germany and other countries [Reference MacDonald23–Reference Allaby and Mayon-White26]. In outbreak situations, contact persons often test positive for other STEC strains than the outbreak strain. Our findings do not support that there is a high prevalence of asymptomatic STEC in the general population. Still, little is known about secondary transmission by asymptomatic carriers [Reference Abu-Sin27]. Furthermore, from our own laboratory data (unpublished data) we know that contact screening sometimes detects asymptomatic children carrying STEC strains other than the outbreak strain suggesting that different serotypes circulate during outbreaks. Contact persons of STEC cases might have been exposed to similar risks as the cases, such as having consumed the same food products that constitute a risk for STEC infection.
In Norway, the National Institute of Public Health bases its decision about exclusion from school on the clinical severity of the STEC infection and the age of the child [28]. If a child in a nursery setting is diagnosed with stx1-producing E. coli, which causes less severe symptoms, the child should stay at home until two stool samples test negative. If a child is diagnosed with stx2-producing STEC or other serotypes which are more likely to trigger HUS, readmission requires five consecutive negative tests [Reference MacDonald23]. Snedeker et al. [Reference Snedeker24] showed with an international systematic descriptive and statistical analysis (literature search and generalized linear models) that 19% of E. coli O157 outbreak cases were the result of secondary spread. Secondary outbreak cases were significantly younger (median age of <6 years vs. 6–16 or 17–59 years) than primary outbreak cases and the number of secondary cases via person-to-person transmission was statistically higher in nurseries than for home or other settings. It might be worth considering a similar approach in Germany, taking into account epidemiological characteristics, such as age of children and microbiological characteristic of the bacteria (stx type 1 and/or stx type 2, eae and sorbitol fermentation).
Prevalence of ESBL-E
Our finding of an ESBL-E prevalence of 2·3% (95% CI 0·3–4·2) in asymptomatic children in Northern Germany corresponds well with the results of other studies performed in Sweden (2·9%) [Reference Kaarme29], Portugal (2·7%) [Reference Guimaraes30] and France (4·6%) [Reference Birgy31].
Several exposures have been shown to increase the risk for ESBL-E carriage including foreign travel, having contact with pets [Reference Meyer32], person-to-person transmission [Reference Haller16], hospital stay [Reference Tham33] and antibiotic treatment [Reference Livermore and Woodford12]. A number of studies found ESBL-producing E. coli in food production animals and companion animals [Reference Valentin34–Reference Geser, Stephan and Hächler36].
Livermore & Woodford [Reference Livermore and Woodford12] showed that intake of antibiotics is a risk factor for ESBL-E carriage. According to the European Antibiotic Resistance Surveillance System (EARS-Net [37]), the proportion of E. coli that are resistant to third-generation cephalosporins, an indicator of ESBL production, has been increasing across Europe during the last decade. In 2013, the population-weighted mean percentage for third-generation cephalosporin resistance in Europe was 12·6%, ranging from 5·0% (Iceland) to 39·6% (Bulgaria). In 2014, Germany reported a proportion of 12·6% of isolates resistant to third-generation cephalosporin (cefotaxim) [38]. The proportion for third-generation cephalosporin resistance in Lower Saxony was 11·1% for 2014 and has increased over the last years [39]. Our study showed that ESBL-E carriage was associated with antibiotic treatment in the preceding 6 months. The situation is worrying because of the high proportion (70%) of antibiotic prescription in children aged <5 years [14].
The high frequency of raw milk consumption and its positive association with ESBL-E carriage in asymptomatic children is notable. Raw milk is not heat-treated and may contain pathogens (e.g. Campylobacter, Listeria, Mycobacterium bovis, STEC, Salmonella or norovirus [Reference Headrick40]). In Germany, the sale of raw milk is strongly restricted. Farms that are allowed to sell raw milk need a licence and must undergo strong quality control procedures. Besides this, farms are allowed to sell milk directly to persons in the neighbourhood if they explicitly advise the customers to boil the milk before consumption. However, to date there have been relatively few reports regarding ESBL-E in raw milk. Skočková et al. [Reference Skočková41] evaluated the presence of ESBL-E. coli in bulk tank milk and found only two (0·7%) out of 270 isolates positive. Geser et al. [Reference Geser, Stephan and Hächler36] did not find ESBL-E in tank bulk raw milk, while Rasheed et al. [Reference Rasheed42] detected a higher proportion of drug-resistant E. coli in raw milk (6·7%, 2/30) but did not detect ESBL-E. coli.
In our study, we found that ESBL-E carriage was significantly associated with having contact with pet rodents. Knowledge about ESBL-E prevalence in pets is limited but a few studies showed that ESBL-producing E. coli strains are commonly found in dogs, cats, horses [Reference Ewers43] and cattle [Reference Schmid44]. Guenther et al. [Reference Guenther45] reported that urban rats (16% out of 56) carried an ESBL-producing E. coli strain and might be a potential threat for humans acquiring ESBL-E, as the rats might contaminate the environment. Ho et al. [Reference Ho46] reported a carriage rate of ESBL-producing E. coli of 4·2% (out of 456 rodents) in wild rodents (unspecified). Taken together, these results indicate that pet rodents are a potential source for ESBL-E infection in humans.
Our study has some limitations. All identified potential risk factors should be considered with caution as our numbers are small and the confidence intervals of the corresponding odds ratios are wide. Moreover, there is the possibility of not identifying true risk factors due to small numbers of carriers. It is also possible that different risk factors exist for the three different Enterobacteria strains (e.g. Klebsiella, Citrobacter, E. coli). There might also be a selection bias in the recruitment of nursery children as we performed convenience sampling and provided the information material only in the German language.
CONCLUSION
In conclusion, the low STEC prevalence in asymptomatic children suggests that exclusion policies may successfully contribute to reducing transmission in nurseries. Nevertheless, a staged approach to exclusion of asymptomatic children should be considered based on their age and the found stx gene type in order to limit unnecessary socioeconomic impact. More research should be conducted on the effect of secondary transmission through asymptomatic carriers in nurseries. This study was performed to gain initial primary data for hypothesis generation.
Our study revealed a low prevalence of ESBL-E. It is, nevertheless, a matter of concern, since there is a reservoir of antibiotic-resistant genes within the community. Further epidemiological studies should focus on the characteristics of ESBL-E in pets and raw milk to gain a better understanding of these potential risk factors in asymptomatic children. Parents should be more intensively informed about the increased risk of foodborne illness from drinking raw milk especially for vulnerable groups, such as infants.
ACKNOWLEDGEMENTS
We sincerely thank colleagues from the local public health departments (Dr Katharina Hüppe (Hildesheim), Dr Carsten Rieck (Cuxhaven), Dr Brigitte Buhr-Riehm (Braunschweig), Dr Harald Speck (Wesermarsch) and Dorothee Maedge (Stadt Braunschweig) for recruiting nurseries in their districts. We are grateful to Sandra Heidrich, Ina Holle and Nicola Jahn for developing the database and entering the data. We thank our colleagues at the NLGA microbiology laboratory for excellent technical assistance and Katja Claußen for supervising the laboratory analyses. Our thanks also go to all participating parents and children. A special thank you goes to the European Programme for Intervention Epidemiology Training and the Postgraduate Training for Applied Epidemiology (Katharina Alpers, Christian Winter and Irina Czogiel) without whose efforts and commitment this work during the past two years would not have been possible. Finally, yet importantly, we thank Emily MacDonald for reading the manuscript.
DECLARATION OF INTEREST
None.




