Streptococcus equi subsp. zooepidemicus (S. zooepidemicus) belongs to the group C streptococci, which are recognized as either commensals or pathogens in a wide array of domesticated animal species, as well as occasional pathogens in humans [Reference Johnson, Tunkel, Mandell, Bennett and Dolin1]. S. zooepidemicus is a large colony-forming species of the group C streptococci (also including S. dysgalactiae, S. equisimilis, S. equi) that produces a streptolysin S haemolysin responsible for β-haemolysis on blood agar but not streptolysin O, and ferments sorbitol, but not trehalose. Group C streptococci are not considered part of the normal human flora [Reference Johnson, Tunkel, Mandell, Bennett and Dolin1, Reference Deibel, Seeley, Buchanan and Gibbons2]. Many human infections can be traced to an animal source or consumption of unpasteurized dairy products, and it has been suggested that S. zooepidemicus is potentially more virulent than other subspecies of the group [Reference Bradley3, Reference Barnham, Thorton and Lange4]. About 30 cases of group C streptococcal meningitis have been reported in the literature, which include those cases caused by S. zooepidemicus [Reference Shah, Matthews and Cohen5, Reference Ural6]. In reviewing the literature in English and Spanish, we were able to find 19 cases of meningitis specifically caused by S. zooepidemicus (Table 1), in addition to the case we describe. As previously reported, this appears to be the most common subspecies of group C Streptococcus to cause meningitis [Reference Shah, Matthews and Cohen5].
Table 1. Cases of human meningitis caused by S. zooepidemicus
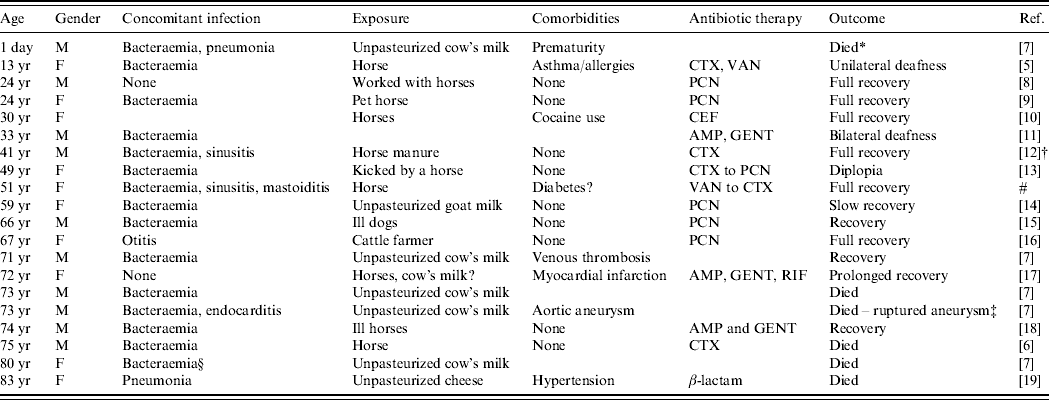
AMP, Ampicillin; CEF, cefotaxime; CTX, ceftriaxone; GENT, gentamicin; PCN, penicillin; RIF, rifampin; VAN, vancomycin.
Blank spaces indicate missing information.
* Died from respiratory distress.
† Lumbar puncture, history, and examination consistent with meningitis, but CSF was culture negative.
‡ Unable to isolate S. zooepidemicus from aneurysm.
§ Not clear if S. zooepidemicus was isolated from CSF, but had meningitis during an outbreak.
# Present study.
Nearly all of the S. zooepidemicus meningitis cases analysed had a zoonotic exposure. Horses were implicated in most cases of direct animal contact, and unpasteurized dairy products were a food source in several reports, some corresponding to a known outbreak of S. zooepidemicus [Reference Edwards, Roulson and Ironside7]. In five instances, illness (mostly respiratory) was reported in the source animals prior to the clinical manifestation of meningitis in humans. Some case-reports and case-series provided minimal clinical details, yet some characteristics merit mentioning from cases with more extended descriptions. The period of incubation in humans ranged from 1 to 21 days (median 7 days). When clinical details were provided, fever was present in the majority of cases, as was the presence of meningeal signs or symptoms (including caephalgia, stiff neck, emesis and/or photophobia). Indeed, changes in mental status at presentation, including unconsciousness and stupor, were reported in nine cases. White blood cell counts (WBC) ranging from 14·7 to 40·8 cells/mm3 were reported from laboratories. In cerebrospinal fluid (CSF), pleocytosis was observed (520–6000 cells/mm3), with neutrophilic preponderance in all cases, and with hypoglycorrhachia as low as 0·5 mg/dl and increased proteinorrhachia also reported in almost all cases where laboratory details were provided. Few cases reported Gram stain, with about 50% of the cases being positive. In a previous review, Shah et al. found an all-cause mortality rate of 43% (12/28 cases) for patients with group C streptococcal meningitis [Reference Shah, Matthews and Cohen5]. Of the cases of S. zooepidemicus meningitis reviewed by us, the mortality rate was 30% (6/20 cases, including ours), even though 75% of patients had documented concomitant bacteraemia. Moreover, at least 45% of these patients had previously been healthy, with a lower rate of comorbidities compared to reports of 73% comorbidity found in patients with group C streptococcal bacteraemia [Reference Bradley3]. However, the occurrence of comorbidities may have been underreported in the cases that we reviewed. Ural et al. mention that S. zooepidemicus infection occurs mainly in healthy individuals [Reference Ural6]. This could be the clinical reflection of the virulence of the organism. However, all of those who died were at the extremes of age, which is consistent with prior reports of group C streptococcal meningitis [Reference Shah, Matthews and Cohen5].
As is the case for other β-haemolytic streptococci, penicillin remains the treatment of choice for all group C streptococci, despite the finding that penicillin may kill S. zooepidemicus more slowly than other group C Streptococcus subspecies [Reference Barnham, Ljunggren and McIntyre20]. The favourable outcome with penicillin in the cases we reviewed clearly shows that the clinical significance of this is unclear. There is reported increased bactericidal activity when gentamicin is used in combination with penicillin [Reference Rolston, Chandrasekar and LeFrock21]. Salata et al. suggest a trend towards improved clinical outcome when penicillin is used in combination with aminoglycosides for severe infections, but acknowledge that the patient sample size prohibits any definitive conclusion [Reference Salata22]. In their review, Shah et al. reported that 6/11 patients with group C streptococcal meningitis who received combination therapy of a β-lactam+aminoglycoside died, providing further confounding evidence regarding the addition of aminoglycosides in the treatment of group C streptococcal meningitis [Reference Shah, Matthews and Cohen5].
We recently cared for a 51-year-old female who started with a mild upper respiratory tract infection 2 weeks prior to admission. Two days prior to hospitalization, she developed otalgia. She was prescribed ciprofloxacin for presumed acute otitis media; the symptoms persisted for 2 days on this antibiotic (unknown dosage), until the next morning, when she was found minimally responsive by her mother and was rushed to the local community hospital. Upon initial evaluation, she was febrile at 40·2°C and stuporous. Her WBC was 17·4 cells/mm3. Lumbar puncture revealed cloudy fluid with an opening pressure of 37 cmH2O, containing 2140/mm3 leukocytes, 823 mg/dl protein, and 14 mg/dl glucose. There was no differential immediately available to us on the CSF white count. Gram-positive cocci in chains were found. Corresponding venepuncture showed leukocytosis and a serum glucose level of >400 mg/dl. She was given 10 mg i.v. dexamethasone, 1 g vancomycin, 2 g ceftriaxone, and 1 g acyclovir before transfer to our facility for further management. The patient's mother initially reported no significant past medical history for her daughter, except for a severe penicillin allergy. At our facility, the patient was unresponsive and in severe respiratory distress with tachypnoea (37 respirations per minute) and a saturation of haemoglobin of 95% while breathing on room air; she was intubated immediately. On physical examination she was an obese female, not requiring pressors; her temperature was 39·1°C and blood pressure 199/105 mmHg. Right middle ear effusion and marked nuchal rigidity were noted, while pupils were symmetrically reactive. No cardiac murmurs were found, and her lungs were clear to auscultation. Abdomen was soft without noticeable organomegaly. No gross focal neurological deficit was noted. The remainder of her examination was unremarkable. Computed tomography of the head and sinuses revealed pansinusitis and bilateral mastoiditis, without involvement of the middle ear or evidence of direct intracranial extension of the infection. She was continued on vancomycin and ceftriaxone at our facility until day 2 of hospitalization, when the isolate was confirmed as group C Streptococcus by Lancefield antigen testing at the community hospital. The isolate was penicillin sensitive with a minimum inhibitory concentration of 0·047 mg/l, but given the patient's reported penicillin allergy, the decision was made to complete a 14-day course of ceftriaxone (2 g i.v. every 12 h). Isolates from both blood and CSF were later determined to be Streptococcus equi subsp. zooepidemicus. Right middle ear myringotomy performed on day 2 of hospitalization showed no growth, and a bronchoalveolar lavage on the same day (performed due to a radiographic image of an unspecific infiltrate) was unremarkable as well. Her blood cultures were negative at our facility, and she was eventually extubated on day 7 of hospitalization. Her mental status improved with no residual neurological deficits. Our patient, for who no past medical history was reported, probably had undiagnosed diabetes mellitus, a comorbidity reported in 20% of patients with group C streptococcal infections [Reference Salata22]. The patient's mother denied any exposure to animals or unpasteurized dairy products at the time her daughter was transferred to our facility. Upon further questioning, she eventually recalled that her daughter began horseback riding at a friend's farm 4 weeks prior to onset of her initial upper respiratory tract symptoms. Unfortunately, the strain isolated from the patient was not available for typing from the outside hospital by the time we requested it.
In summary, S. zooepidemicus should be considered in patients presenting with acute onset meningitis after contact with animals, particularly horses. It can also be acquired by consumption of unpasteurized products, which can occur in outbreaks as well. Monotherapy with penicillin remains the antibiotic of choice.
ACKNOWLEDGEMENTS
The authors thank Robert Muder, M.D., for his kind review and useful suggestions for the final drafting of the manuscript.
DECLARATION OF INTEREST
None.



