INTRODUCTION
Ornithosis is a zoonotic disease caused by the bacterium Chlamydophila psittaci (C. psittaci) that is mainly transmitted from birds to humans [Reference Caul, Sillis, Palmer, Soulsby and Simpson1]. In humans the incubation period is 1–4 weeks and manifestations of the disease can range from asymptomatic infection or mild respiratory illness to pneumonia and fatal systemic illness [Reference Schlossberg, Mandell, Bennett and Dolin2, Reference Heymann3]. People who have had contact with infected birds are at risk of acquiring the disease. However, studies have also suggested that infection may also be acquired indirectly by inhalation of dust contaminated with infected bird droppings without a history of direct contact with birds [Reference Williams4]. Infected birds are often asymptomatic or have non-specific signs such as lethargy, anorexia, or ruffled feathers [Reference Smith5].
Outbreaks of ornithosis are known to occur in poultry farms and abattoirs. These outbreaks have commonly been on duck and turkey farms and in abattoirs [Reference Newman6–Reference Hinton10]. Studies have found that evisceration workers have the highest risk for acquiring ornithosis [Reference Hedberg7, Reference Andrews, Major and Palmer11]. There is less information available about the risk of ornithosis-related pneumonia and work area.
In the state of Victoria, Australia, medical practitioners and laboratories are required by law to notify health authorities of human cases of ornithosis [12]. In November 2003, a case of ornithosis in a commercial duck abattoir worker was notified to the Communicable Diseases Section of the Victorian Government Department of Human Services, Australia. More cases were subsequently identified which led to a serological survey and case-control study at the abattoir and onsite duck farm. Our investigation was primarily focused on human health and was conducted in order to (1) identify the extent of exposure to the C. psittaci at the worksite, and (2) identify high-risk work areas that were associated with ornithosis-related pneumonia to guide prevention strategies. Results of environmental and veterinary investigations are also described to provide context for the human outbreak.
METHODS
Epidemiological investigations
Passive surveillance for ornithosis was enhanced by sending health alerts to medical practitioners in the area surrounding the duck farm, as well as to all workers. Cases notified to the Communicable Diseases Section were classified as confirmed or probable according to the Communicable Disease Network of Australia case definitions for ornithosis [13]. A confirmed case was a person who (i) had a four-fold rise in antibody titre against C. psittaci, and (ii) clinical evidence of either pneumonia or at least two of the following symptoms: fever, headache, myalgia, rigors, dry cough or dyspnoea. Probable cases were persons who had clinical evidence of infection and an elevated titre to C. psittaci.
The epidemiological investigation was conducted in two parts. First, a serological survey was performed in order to find out the extent of exposure to C. psittaci and second, a case-control study was conducted to ascertain the risk factors associated with acquiring ornithosis-related pneumonia. As these studies formed part of an outbreak investigation and response, ethical approval was not required.
A site visit was organized in May 2004 and all employees including abattoir, farm, maintenance and office workers who were present during a 3-day period were asked to participate. Those who agreed were interviewed by department staff using a standard questionnaire and had a single blood sample taken for C. psittaci IgG levels. Workers were asked to recall prior clinical symptoms between 1 October 2003 and 30 April 2004 that included any two of the following: fever, headache, myalgia, rigors, dry cough or dyspnoea. They were also asked about their length of employment, their areas of work, whether they smoked, their general health status and lawnmowing activity, the latter being a risk factor identified in a previous outbreak [Reference Williams4]. Abattoir workers were specifically asked about which of the six areas of the abattoir building they had worked.
An in-house algorithm that consisted of a two-level approach for the assessment of serological results was used to assign seropositivity. All samples were initially tested using an enzyme immunoassay for the detection of IgG antibodies to the Chlamydophila genus (Savyon SeroELISA Chlamydia IgG; Savyon Diagnostics Ltd, Ashdod, Israel, cat. no. 111-01). Experienced laboratory scientists performed a second round of testing using an immunofluorescence test (Savyon SeroFIA IgG, Savyon Diagnostics Ltd, cat. no. 511-01M) to confirm the presence of C. psittaci IgG antibodies on samples that had an optical density >1·0 on enzyme immunoassay. The enzyme immunoassay test was less subjective and allowed screening of a large number of samples for the presence of Chlamydophila genus antibodies, while the immunofluorescence test allowed for the differentiation of Chlamydophila species. A cut-off of 1·0 for the enzyme immunoassay was chosen as a result of in-house testing that found that previous serum samples that were positive for C. psittaci on immunofluorescence had optical densities >1·0 using enzyme immunoassay on convalescent samples (average optical density was 2·3). We considered titres of ⩾64 as seropositive to C. psittaci, in accordance with the manufacturer's instructions. Samples with titres of ⩾512 for C. pneumoniae were considered to represent significant cross-reactions.
For the case-control study, cases were defined as workers who were notified with radiologically confirmed pneumonia and a C. psittaci IFAT IgG titre of ⩾64. All cases had their onset of illness between 1 October 2003 and 30 April 2004. We sought controls from the same occupational group as cases. This included workers who were present during the site visit plus additional workers who were identified during the outbreak investigation, including former workers and current employees who were not present during the site visit. We attempted to maximize the power of the study by obtaining as many controls as possible. Both cases and controls were interviewed using the same questionnaire as in the seroprevalence study.
Data were analysed using stata version 8.0 [14]. Univariate analysis was performed using exact 95% confidence intervals (CI) and χ2 tests, while the Wilcoxon rank-sum test was used to compare age distributions. Fisher's exact two-sided P value was used when expected cell counts were <5. To differentiate the areas of work where employees were most likely to acquire pneumonia, an unconditional logistic regression model was used to control for possible confounding by multi-area workers and length of employment. Further cases of pneumonia in employees were notified to the department in June and July 2004. The case-control study was analysed with and without these cases.
Environmental investigations
Environmental investigations were conducted in February and March 2004. The layout and work practices at both the abattoir and farm were examined for possible perpetuating causes of infection.
Veterinary investigations
Site inspections of the farm were conducted and samples were taken from ducks for culture and serology. Eye and throat swabs for culture were taken in December 2003 and March 2004, while fortnightly blood samples for antibody testing using a modified complement fixation test (CFT), previously validated in other avian species [Reference Grimes and Page15] were taken prior to slaughter during this period. Flocks with a titre of 1:8 using this test were considered likely to have been infected with C. psittaci.
RESULTS
Epidemiological investigations
Nineteen cases of ornithosis were notified by the end of April 2004 (Fig. 1). During this same period there were no other notifications of ornithosis from the surrounding district.

Fig. 1. Cases of ornithosis from a commerical duck abattoir and farm notified to the Victorian Government Department of Human Services, Australia. □, Probable cases; ■, confirmed cases.
Serological survey
There were 126 workers present during the 3 days of the investigation. A total of 97 (77%) workers were interviewed and had blood taken during this time. Of the 97 workers, 53 (55%) were seropositive to C. psittaci. The majority of these seropositive workers (35, 66%) did not have a cross-reaction with C. pneumoniae (Table 1).
Table 1. The number of workers with significantly elevated titres to C. psittaci compared to C. pneumoniae

* Indicates infection at indeterminate time.
† Indicates current or recent infection.
Seropositive workers were older than the seronegative group [median age 43·0 (range 17–63) years vs. 32.5 (range 19–69) years; P=0·003]. There was no difference in the sex distribution between the two groups [odds ratio (OR) 0·66, 95% CI 0·26–1·6). Workers who had been at the company for >7 months had greater odds of being seropositive (OR 2·8, 95% CI 1·1–7·1) and current smokers were more likely to be seronegative (OR 0·3, 95% CI 0·1–0·8). There was no association between being seropositive and history of chronic respiratory problems (OR 1·2, 95% CI 0·4–4·2), contact with domestic or wild birds outside the company (OR 1·3, 95% CI 0·5–3·6) or mowing lawns (OR 1·6, 95% CI 0·7–4·0).
The occupational group that had the highest percentage of employees who were seropositive was abattoir workers (65%) followed by maintenance workers (47%), farm workers (42%) and office workers (17%) (Table 2). Of those who were seropositive, the percentage reporting symptoms consistent with the clinical case definition ranged from 0% to 54% in the different occupational groups (Table 2). Within the abattoir, employees who had worked in the slaughtering area or kitchen were more likely to be seropositive [14 (70%) and 14 (70%) respectively] compared with 12 (67%) in the gutting area, 21 (66%) in the grading area, four (57%) in the hanging area and five (56%) in the dispatch area. These differences were not statistically significant (all P>0·05).
Table 2. Number of workers seropositive and seropositive with clinical symptoms according to occupational group in a serological survey of an outbreak of ornithosis at a commercial duck abattoir and farm
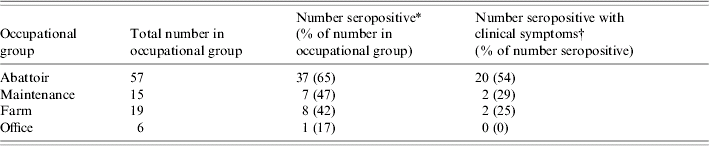
* Seropositve was defined as a titre of ⩾64 to Chlamydophila psittaci, detected on immunofluorescence.
† Clinical symptoms were defined as any two of: fever, headache, myalgia, rigors, dry cough or dyspnoea occurring while working at the site between 1 October 2003 and 30 April 2004.
Analysis excluding the 18 cases with a significant cross-reaction to C. pneumoniae was also performed. Similar results were found when this group was compared to seronegative employees, with the only significant association being age (P=0·003) and working at the company for >7 months (OR 2·9, 95% CI 1·0–8·6). The occupational group with the highest percentage of employees were again abattoir workers (54%) followed by maintenance workers (43%), farm workers (27%) and office workers (17%).
Case-control study
Between 1 November 2003 and 30 April 2004 there were ten workers notified who had radiological evidence of consolidation or pulmonary infiltrates and who were included as cases. A further two workers developed pneumonia with radiological evidence of consolidation after the study period and were included in parts of the analysis. All who developed pneumonia were abattoir workers. There were 61 controls consisting of 54 abattoir workers who were interviewed during the site visit, six former workers and one part-time worker identified during the outbreak investigation. None of the controls had a history of radiologically confirmed pneumonia in the 7 months prior to the case-control study.
The 12 cases all had fever, 11 (92%) had a cough, 10 (75%) had sweats and chills, 10 (75%) had myalgia, nine (75%) had a headache and six (50%) had dyspnoea at the time they had pneumonia. Eight (67%) cases had a four-fold increase in C. psittaci IgG, two (17%) had a two-fold increase and two (17%) cases had single titres ⩾64. Five (42%) cases required hospitalization and the median length between starting work to onset of illness was 14 days (range 8–76 days).
Univariate analysis including the ten cases identified during the initial study period found that controls were more likely to have worked in the abattoir for >7 months (OR 0·06, 95% CI 0·001–0·5). There were no significant associations between cases and controls with respect to the presence of chronic respiratory problems (OR 0·34, 95% CI 0·0–2·9), smoking (OR 1·1, 95% CI 0·21–5·22), having contact with birds outside the company (OR 0·6, 95% CI 0·06–3·44) or mowing the lawn (OR 0·16, 95% CI 0·0–1·3). Cases and controls did not differ in terms of age [median age 37 (range 17–56) years vs. 36 (range 19–69) years; P=0·93] and sex distribution (OR 2·2, 95% CI 0·44–14·6). There were, however, more former workers among the cases (OR 9·5, 95% CI 1·3–63·5).
There were cases in all areas of employment in the abattoir (Table 3). Univariate analysis for the ten cases identified during the initial study period found that those who had pneumonia were more likely to have worked in the slaughtering area (OR 6·0, 95% CI 1·2–39). There was no significant association with pneumonia and other areas of work (all P>0·05). Multivariate analysis, which took into account possible confounding by multi-area work and length of employment, suggested that those with pneumonia were more likely to have worked in the slaughtering area [adjusted OR (aOR) 16·7, 95% CI 1·3–207] (Table 4).
Table 3. Work area of cases of radiologically confirmed pneumonia among abattoir workers in a case-control study of an outbreak of ornithosisFootnote *
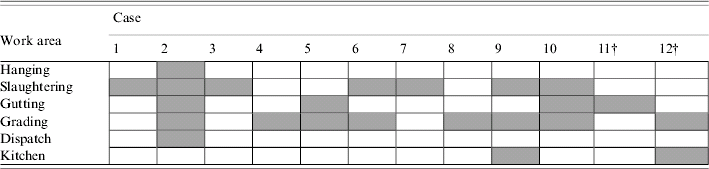
* Areas worked by cases <30 days prior to onset of illness indicated by grey boxes.
† Cases 11 and 12 were identified after the initial study period.
Table 4. Multivariable analysis for the odds of acquiring pneumonia according to work area among abattoir workers in a case-control study of an outbreak of ornithosisFootnote *

OR, Odds ratio; CI, confidence interval.
* χ2 for covariates=24·2 (7 d.f.), P=0·001.
† Odds ratio adjusted for work in other areas and length of employment.
When the two cases of pneumonia identified after the study period were included in the analyses using a multivariable model for work area and working for >7 months, the latter remained significantly protective (aOR 0·05, 95% CI 0·0–0·45). The highest odds of pneumonia associated with the slaughtering area remained, although this was no longer significant (aOR 4·55, 95% CI 0·7–29·3). All other areas were not significantly associated with pneumonia (all P>0·05).
Environmental investigations
Workers at the site were divided into four broad occupational groups: abattoir, farm, maintenance and office. Abattoir workers were involved in the slaughtering and processing of the ducks while farm workers were involved with the handling of live animals. Maintenance workers moved around the abattoir buildings and growing sheds but had little contact with the birds. Lastly, movement of office workers was limited to amenities buildings that were adjacent to the abattoir building and growing sheds.
Within the abattoir there were six separate rooms or work areas: hanging, slaughtering, gutting, grading, dispatch and kitchen (Fig. 2). Ducks from the farm were transported to an outside hanging area where workers shackled the live birds on a moving line. The ducks were moved into an indoor slaughtering area where they were slaughtered and de-feathered. In this area, workers manually slaughtered the ducks at close range while de-feathering was performed both manually and mechanically. There was heavy contamination of the environment with blood and feathers. Machines were used to eviscerate the carcasses in a separate room before they were moved into the gutting room where workers packaged the heart and kidneys. The carcasses were then passed into a grading room where workers sorted the meat into different grades and removed the bones. Finally the carcasses were passed into cold storage in the dispatch area or the kitchen for cooking prior to marketing. Within the abattoir workers are often moved between the various work areas.

Fig. 2. The floor plan of an abattoir involved in an outbreak of ornithosis. Indicated are main work areas and process flow (arrows). Evisceration was performed mechanically in this plant.
Veterinary investigations
Ducks of different ages were housed together in 13 growing sheds that were open and unprotected from wild birds. Investigations found that flocks were infected with both Riemerella anatipestifer, cultured from airsac swabs, and C. psittaci, confirmed by culture from nasopharyngeal swabs and positive serology tested by CFT in December 2003. Despite being on antibiotics (oxytetracycline in feeds up to 3 weeks of age, in water at 5 weeks and withholding of antibiotics 1 week before slaughter at 6 or 7 weeks) since December 2003, the ducks remained positive for C. psittaci on repeated culture in March 2004 (M. Lancaster personal communication, May 2004).
DISCUSSION
In this outbreak of ornithosis in a commercial duck abattoir and farm, we found multiple sites where workers showed serological evidence of exposure to C. psittaci. This included workers who did not have direct contact with poultry, such as maintenance and office workers, as well as those who did, such as abattoir and farm workers. We investigated the association between those who had radiologically confirmed lower respiratory tract infection to identify high-risk work areas. All workers who had ornithosis-related pneumonia worked in the abattoir. While cases of pneumonia were distributed throughout the various work areas within the abattoir, those who were most susceptible to illness appeared to be new employees and those working in the slaughtering area where slaughtering and de-feathering of carcasses occur (aOR 16·7, 95% CI 1·3–207).
Our finding that longer-term workers were more likely to be seropositive (OR 2·8, 95% CI 1·1–7·1) and less likely to have pneumonia (OR 0·06, 95% CI 0·001–0·5) suggests that the organism may have infected flocks for a longer period than was evident from notifications. Investigation of similar outbreaks found that long-term employees were more likely to be seropositive, while new workers were more likely to have ornithosis [Reference Newman6, Reference Hinton10]. This suggests that ornithosis may be a long-term problem in the poultry industry and that long-term employees may develop some immunity, while new workers are susceptible to infection.
This study had limitations. Seropositivity in the workers may have a number of interpretations. Exposure to the organism could have occurred in the workplace or outside the workplace and false-positive antibody results due to cross-reactivity with other Chlamydophila species or non-Chlamydophila species are another possibility. However, from November 2003 to April 2004, apart from cases at the worksite, there were no other notifications of ornithosis from this region. Furthermore, there was no statistical difference in the seropositive and seronegative groups when alternative forms of exposure such as exposure to birds outside the company and lawnmowing were analysed. False-positive results remain a possibility, although cases were tested using the SeroFIA immunofluorescence test kit, a specific and sensitive serological test for differentiating Chlamydophila species [Reference Russell16] and a high cut-off was used in the initial screening test to minimize false-positive results. As expected, a number of workers seropositive for C. psittaci also had reactions with C. pneumoniae, leaving a degree of uncertainty about the causative agent. However, the majority of seropositive workers (66%) did not have significant cross-reactions with C. pneumoniae, and the exclusion of cases that cross-reacted with C. pneumoniae would not have altered the conclusions of this study.
The diagnosis of pneumonia depended on physicians ordering appropriate investigations. Cases may have been missed during the outbreak investigation. However, staff and management were notified of the problem in December 2003 and health alerts were sent out to all medical doctors in the area, making this event less likely. Other possible causes of pneumonia were not sought. However, ornithosis appears to be the most likely cause of pneumonia as 10 (83·3%) showed rising serological titres to C. psittaci, and infection in poultry was proved by the isolation of C. psittaci from nasopharyngeal swabs.
While working in the slaughtering area was found to be a significant risk factor for the original study period, the addition of cases prospectively reduced this association. It should be noted, however, that prospective changes in the control group, such as the number of new workers allocated to certain work areas, could not be taken into account for this analysis. It may be that working for <7 months at the abattoir confounded the relationship between slaughter area and risk of pneumonia due to the usually high turnover of staff in this work area.
Several risk factors may have accounted for the increased likelihood of pneumonia in those working in the slaughtering area. First, the slaughtering process was performed manually at close range. Ornithosis is a known to be a systemic disease in birds, infecting blood and the major organs including the lungs, liver, spleen, and kidneys [Reference Anderson and Vanrompay17]. Therefore, holding birds at close range and severing the trachea could potentially release large amounts of organism. A large amount of blood aerosols may be released during slaughtering and could be responsible for transmission. Second, the slaughtering area was an enclosed environment that was heavily contaminated with blood and feathers. C. psittaci is able to survive for several months under the cover of organic material [Reference Caul, Sillis, Palmer, Soulsby and Simpson1]. Heavy contamination of this area may therefore result in survival of the organism and increased opportunity for infection of workers. Our findings differed from other studies which found that evisceration posed the highest risk of ornithosis for workers [Reference Hedberg7, Reference Anderson, Stoesz and Kaufmann9, Reference Andrews, Major and Palmer11]. However, in this abattoir evisceration was predominantly performed mechanically. Like other outbreaks we found that the risk of illness and exposure to the organism was widespread throughout the site. Employees who did not work in the slaughtering area also developed pneumonia. It may be possible that other processes such as de-boning or cutting the meat may also release the organism into the air. Other authors have also suggested that alternative forms of transmission, such as through the skin, may be responsible for transmission in these workers, although this has not been verified [Reference Hedberg7].
While farm workers had close contact with poultry, there were no workers who developed ornithosis-related pneumonia and there was a smaller proportion of workers who were seropositive. It is known that transportation can increase stress and shedding of the organism in birds [Reference Smith5]. This may have contributed to increased bacterial load in birds that were brought to the abattoir, in addition to the release of organisms from internal organs during processing.
This outbreak of ornithosis demonstrates that occupational groups moving in the environment are susceptible to illness despite little contact with poultry. However, those susceptible to the development of lower respiratory tract illness are workers in the abattoir who have heavy exposure to blood and feathers. Preventative measures during outbreaks should encompass these risks. This includes providing workers with information regarding the signs and symptoms of ornithosis, respiratory protection all for abattoir workers and consideration of environmental changes to high-risk areas, such as improvements to airflow and reduction of environmental contamination.
Ultimately, control of infection in humans requires reducing the bacterial load in animals. Despite antibiotic treatment of the flocks, infection continued, and various factors may have been responsible for perpetuating the infection. Different cohorts of birds were mixed together and may have allowed re-infection during periods when antibiotics were withheld prior to slaughter. Ducks were also housed in open sheds and wild birds may have been responsible for the introduction and continued infection of flocks. Therefore, in order to control the development of further human cases of ornithosis, control of infection of birds through changes to farming practices is imperative.
ACKNOWLEDGEMENTS
Thanks are due to: Stephen Waddington, Jonathon Ehsani, Pauline Lynch and Marion Moloney for data collection; Simon Madin, Rosemary Lester and Mary Deeble for editorial advice; and Malcolm Lancaster for veterinary advice.
DECLARATION OF INTEREST
None.







