Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
De St Groth, S. Fazekas
1952.
Nasal mucus and influenza viruses. II. A new test for the presumptive diagnosis of influenza infection.
Epidemiology and Infection,
Vol. 50,
Issue. 4,
p.
491.
Springer, Georg F.
1955.
�ber fucosehaltige Mucine vorwiegend entodermalen Ursprungs mit Blutgruppen- und anderen biologischen Eigenschaften.
Klinische Wochenschrift,
Vol. 33,
Issue. 15-16,
p.
347.
Fraser, K. B.
1957.
A latent character of the NWS strain of influenza virus A: The use of glycerol to reveal conversion to the indicator state.
The Journal of Pathology and Bacteriology,
Vol. 73,
Issue. 1,
p.
263.
de St. Groth, S. Fazekas
1963.
Vol. 9,
Issue. ,
p.
1.
Haagen, Eugen
1964.
Viruskrankheiten des Menschen.
p.
59.
Moss, B. A.
and
Underwood, P. Anne
1978.
The Influenza Virus Hemagglutinin.
Vol. 3,
Issue. ,
p.
145.
Yang, Xiaoyun
Steukers, Lennert
Forier, Katrien
Xiong, Ranhua
Braeckmans, Kevin
Van Reeth, Kristien
Nauwynck, Hans
and
Bouvier, Nicole M.
2014.
A Beneficiary Role for Neuraminidase in Influenza Virus Penetration through the Respiratory Mucus.
PLoS ONE,
Vol. 9,
Issue. 10,
p.
e110026.
David, Shannon C.
Vadas, Oscar
Glas, Irina
Schaub, Aline
Luo, Beiping
D'angelo, Giovanni
Montoya, Jonathan Paz
Bluvshtein, Nir
Hugentobler, Walter
Klein, Liviana K.
Motos, Ghislain
Pohl, Marie
Violaki, Kalliopi
Nenes, Athanasios
Krieger, Ulrich K.
Stertz, Silke
Peter, Thomas
Kohn, Tamar
and
Imperiale, Michael J.
2023.
Inactivation mechanisms of influenza A virus under pH conditions encountered in aerosol particles as revealed by whole-virus HDX-MS.
mSphere,
Vol. 8,
Issue. 5,
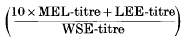 of normal human nasal secretions is 33·2 with a standard deviation of ±7·4. This value is independent of the donor, of the absolute amount of mucus collected, of the time of extraction, and of the period of storage.
of normal human nasal secretions is 33·2 with a standard deviation of ±7·4. This value is independent of the donor, of the absolute amount of mucus collected, of the time of extraction, and of the period of storage.