INTRODUCTION
Pertussis or whooping cough has persisted and resurged in the face of vaccination and has become one of the most prevalent vaccine-preventable diseases in Western countries with estimated infection frequencies of 1–9% [Reference Ward1–Reference Hallander4]. The high circulation rate of Bordetella pertussis poses a threat to infants that have not been (completely) vaccinated and for whom pertussis is a severe, life-threatening, disease [Reference de Greeff5]. The increase in pertussis is mainly found in older age groups in which immunity has waned and this has resulted in the perception that waning immunity is the (exclusive) cause for the resurgence of pertussis.
Estimates of duration of immunity, acquired either by infection or vaccination, vary widely between, respectively, 4–20 years and 4–12 years [Reference Wendelboe6]. This large variation is probably due to different definitions of immunity and different vaccines included in these studies. Mathematical modelling supports a period of natural immunity that is, on average, long-lasting (at least 30 years) but inherently variable [Reference Wearing and Rohani7]. Compared to natural infection, several studies have revealed a shorter duration of protection after vaccination. A modelling study estimated that 15% of individuals vaccinated with an acellular vaccine (ACV), lost their immunity within 5 years after vaccination [Reference Lavine8]. Recent field studies have shown that protection conferred by ACVs is less enduring than previously thought. One study found that, after the fifth dose of an ACV, the odds of acquiring pertussis increased by an average of 42% per year [Reference Klein9]. A second study noted a markedly increased rate of disease from ages 8–12 and vaccine effectiveness was estimated to be 24% in this age category [Reference Witt, Katz and Witt10]. There is also evidence that some whole-cell vaccines (WCVs) induce longer lasting immunity than ACVs, raising the possibility that the switch from WCVs to ACVs may have aggravated the pertussis problem [Reference Gustafsson11, Reference Sheridan12].
Although there is consensus that waning immunity is an important cause for the resurgence of pertussis, we know little about the underlying causes. Such causes include the qualities of the vaccine affecting the immune response, which are determined by adjuvants, pertussis-administered and co-administered antigens. For example, pertussis antigens used in vaccines have immunosuppressive activities in their native form, which may still be (partly) retained in the vaccine, possibly leading to a suboptimal immune response [Reference de Gouw13–Reference Higgs15]. Other factors that may affect waning immunity include lack of natural boosters and adaptation of the pathogen [Reference Lavine, King and Bjornstad16–Reference Mooi, van Loo and King18]. Lack of natural boosters by infection, proposed to be caused by a decrease in the circulation of B. pertussis compared to the pre-vaccination era, is difficult to reconcile with the high (1–9%) infection frequencies observed in the last 10–20 years [Reference Ward1–Reference Hallander4]. Further, if natural infection confers a longer lasting immunity than vaccination, as is widely accepted, one would expect less boosting in the pre-vaccination period compared to the current period if initial immunity is vaccine-induced. The role of pathogen adaptation in waning immunity has been largely ignored. Pathogen adaptation may affect the structure or regulation of B. pertussis products and hence its recognition by, and interaction with, host defences. For example, antigenic variation may diminish the efficacy of antibodies, or affect T-cell recognition and memory. Changes in gene expression, by either up- or down-regulation, may also affect the antigenic profile of the pathogen. If the affected genes code for compounds which modulate host immunity, changes in gene regulation may significantly change pathogen properties. Notably, all these changes have been observed in B. pertussis populations and will be discussed here. We argue that these changes are adaptive and increase strain fitness by decreasing the period in which pertussis vaccines are effective and thus enhance the waning of immunity. Further, the observed changes in B. pertussis populations point to ways of improving vaccines.
Variation in B. pertussis virulence-associated proteins
Identifying genetic polymorphisms is a first step in finding loci important for bacterial adaptation. Early studies on genetic polymorphisms in B. pertussis populations focused on genes coding for proteins known to contribute to immunity: serotypes 2 and 3 fimbriae (Fim2, Fim3), pertactin (Prn) and pertussis toxin (Ptx) (reviewed in [Reference Mooi19] and [Reference He and Mertsola20]). Later, we also included the promoter for Ptx (ptxP) in these studies, in view of the central role we perceived for Ptx in the ecology of pertussis [Reference Schouls21, Reference Mooi22]. Although potentially adaptive mutations have been described in many other genes in later years, when whole genome sequencing became feasible [Reference Octavia23–Reference van Gent25] most was known about these four proteins, both genetically and functionally. Importantly, together with filamentous haemagglutinin (FHA) these proteins are included in acellular pertussis vaccines (ACVs) which have replaced whole-cell vaccines (WCVs) in many countries [Reference Berbers, de Greeff and Mooi26]. Therefore, Fim, Prn, Ptx and ptxP will be the focus of this review. FHA has not been included as little is known about variations in this protein due to the large size of its gene and the presence of repeats which affects the accuracy of sequencing.
B. pertussis strains contain both fim2 and fim3 genes and may express one or both genes [Reference Willems27]. Allelic variation in fim2 and fim3 genes is rather limited. Two Fim2 and three Fim3 variants are found in B. pertussis populations (Fig. 1 a). The Fim3-3 variant has been detected sporadically. As with the other genes discussed here, more alleles than protein variants circulate due to the presence of silent mutations [Reference Mooi19]. Several studies have suggested an important role for fimbrial antibodies in protection [28, Reference Storsaeter29] and care was taken to include strains with both Fim2 and Fim3 in WCVs. Fimbriae are part of two ACVs, the T-type vaccine mainly used in Japan which contains Fim2 in addition to FHA, Ptx and Prn [Reference Kuno-Sakai, Kimura and Watanabe30] and a five-component vaccine which contains both fimbrial serotypes and is used in Europe and North America [Reference Berbers, de Greeff and Mooi26]. With the exception of a Dutch vaccine strain, which contains fim2-2 and fim3-1, all vaccine strains analysed harboured fim2-1 and fim3-1 (Table 1).

Fig. 1. (a) Fim and (b) PtxA variants found in B. pertussis populations. Protein variant designations are shown on the left. Dots indicate identical amino acids. Numbering is relative to the N-terminal methionine. Protein variants found in vaccine strains are underlined. The Fim2-2 variant has only been found in one vaccine strain used in The Netherlands.
Table 1. Protein variants found in pertussis vaccines*

* Adapted from Mooi [Reference Mooi19]. Many ACVs also contain filamentous haemagglutinin which has not been included here as little is known about variation in this large protein.
† Only in one Dutch vaccine strain.
‡ Only in one Swedish vaccine strain.
§ Protein variants found in the strains Tohama I (Prn1, PtxA2) and 10536 (Prn7, PtxA4) used for many acellular vaccines.
Ptx is composed of five subunits (PtxA–E), of which PtxA harbours the toxic activity. PtxA has been shown to be immunodominant over the other four subunits [Reference De Magistris31]. Consistent with this, variation in Ptx is mainly found in PtxA. In B. pertussis populations, five protein variants of PtxA have been identified, PtxA1, PtxA2, PtxA4, PtxA5 and PtxA8, of which PtxA1 and PtxA2 predominate [Reference Mooi19]. Ptx is the only pertussis antigen which has been shown unequivocally to confer protection in humans, since it has been tested as a single component vaccine [Reference Trollfors32]. Indeed, a Ptx monocomponent vaccine has been used in Denmark since 1997 [Reference Hviid33]. However, vaccines with three or more (FHA, Fim, Prn) pertussis antigens in addition to Ptx were found to be more effective than vaccines containing Ptx only, supporting a role for FHA, Fim and Prn in conferring protection against pertussis [Reference Zhang34]. Ptx is incorporated in all ACVs and three protein variants have been found in pertussis vaccine strains, PtxA1, PtxA2 and PtxA4, of which PtxA2 and PtxA4 are produced by strains used for the production of widely used ACVs [Reference Litt, Neal and Fry35] (Table 1).
Seventeen ptxP alleles have been found worldwide of which three (ptxP1, ptxP2, ptxP3) predominate [Reference Mooi22, Reference Advani36–Reference Schmidtke40]. Strains with the ptxP2 allele disappeared after the introduction of vaccination and in current B. pertussis populations mainly ptxP1 and ptxP3 are observed. A comparison of Ptx production showed that ptxP3 strains produced 1·6 times more Ptx than ptxP1 strains [Reference Mooi22]. In contrast, the production of Prn was slightly suppressed in ptxP3 strains compared to ptxP1 strains, suggesting that increased Ptx production was not due to a global up-regulation of virulence genes.
Thirteen Prn protein variants have been identified, of which three (Prn1, Prn2, Prn3) predominate in B. pertussis populations (Fig. 2). Antibodies to Prn have been associated with protection [Reference Storsaeter29, Reference Cherry41]. Strains used for vaccine production contain the prn1, prn7 or prn10 alleles (Table 1). However, the prn10 allele was found in only one Swedish vaccine strain. Although single nucleotide polymorphisms (SNPs) are also present, variation in Prn is mainly found in two regions comprised of five and three amino-acid repeats (regions 1 and 2, respectively; Fig. 2). Variation in repeat units is a mechanism used by many pathogens to escape from host immunity [Reference Madoff42], and it seems likely that the Prn repeats serve a similar function.
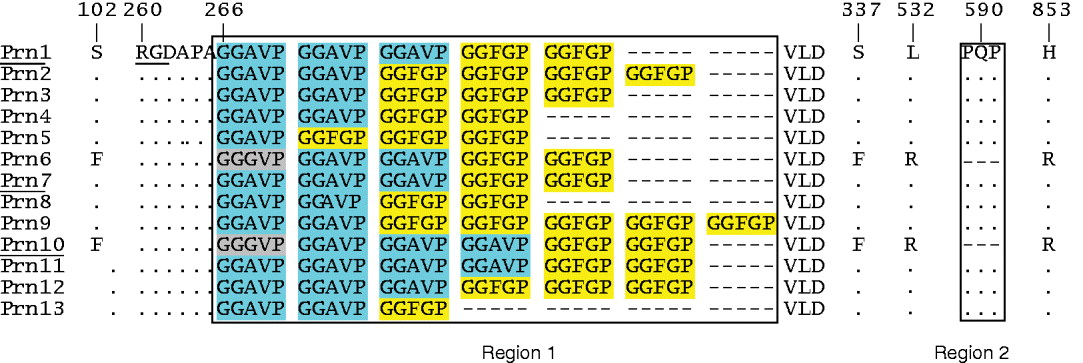
Fig. 2 [colour online]. Prn variants found in B. pertussis populations. Protein designations are shown on the left. Dots and dashes indicate identical and absent amino acids, respectively. Numbering is relative to the N-terminal methionine of Prn1. The two regions (1 and 2) with five and three amino-acid repeats, respectively, have been blocked. The five amino-acid repeats occur as three variants which have been highlighted. The RGD motif involved in adherence, and protein variants found in vaccine strains, are underlined.
Temporal changes in the Dutch B. pertussis population
Analyses of B. pertussis populations in a number of countries have shown that the introduction of vaccination was associated with significant shifts in allele frequencies (reviewed in [Reference Mooi19]). Here we focus on The Netherlands as it offers a number of unique features to the study of the evolution of B. pertussis and in particular to explore the relationship between changes in strain frequencies and notifications. The Netherlands comprises a relatively small country in which vaccination coverage has been consistently high. Further, the vaccines and vaccine strains used have been well characterized, and changes in vaccines and vaccination schedules were implemented nationwide within a short time span. Mass vaccination against pertussis with a WCV was introduced in 1953. In November 2001, an ACV booster was implemented for 4-year-olds and in January 2005 the infant WCV was replaced by an ACV. Two ACVs have been used in The Netherlands, a three-component vaccine from GlaxoSmithKline, containing FHA, Prn and Ptx, and a five-component vaccine from Sanofi Pasteur-MSD which contains two additional antigens, Fim2 and Fim3 [Reference Berbers, de Greeff and Mooi26].
In The Netherlands, we observed the successive appearance of novel, non-vaccine-type, alleles for ptxA, prn, ptxP and fim3 after the introduction of vaccination in 1953. Based on the allelic variation in the four genes, seven allele types could be defined (Fig. 3). Two of these (II and III) are typical for vaccine strains used in The Netherlands and other countries. The remaining allele types are distinct from the vaccine types in one or more of the four genes.

Fig. 3 [colour online]. Relationship between phylogeny and the accumulation of mutations in virulence genes in The Netherlands. The maximum parsimony tree was based on 85 SNPs and 198 Dutch strains isolated between 1949 and 2008. Changes in the alleles for fim3, ptxA, ptxP and prn are indicated. The alleles prn2 and prn3 were combined as they are both non-vaccine types. Based on the four alleles, seven allele types (I–VII) could be distinguished. Coloured dots distinguish allele types and arrows indicate changes between allele types. Whole-cell vaccines used in The Netherlands until 2005 were derived from allele types II and III (blocked). (Adapted from van Gent et al. [Reference van Gent25].)
Phylogenetic analyses based on SNPs of Dutch strains isolated between 1949 and 2008 revealed a tree of which the topology was very similar to that of trees derived for the human influenza A virus haemagglutinin genes, exhibiting a ladder-like structure with a long trunk and short side branches [Reference van Gent43] (Fig. 3). As noted for the human influenza A virus haemagglutinin tree [Reference Bedford, Cobey and Pascual44], the trunk corresponds to the progenitor lineage. Mutations that occur along the trunk are eventually fixed, persisting until replaced by subsequent mutations. In contrast, mutations that appear on side branches are eventually lost from the population. The mutations in four virulence-associated genes fim3, prn, ptxA and ptxP were found in the trunk of the tree and were fixed until they were replaced by novel mutations in the same gene. When travelling from the root to the tip of the tree, a gradual divergence between the two Dutch WCV strains and the B. pertussis population was observed with respect to the four genes. A similar temporal accruement of mutations has been found by Octavia and co-workers [Reference Octavia23] using a geographically more diverse strain collection. The distribution of allele types in the tree indicated that new genotypes emerged de novo, rather than being selected from ancient reservoirs, as reappearance of ancient allele types would be reflected in branches emanating from, or close to, the root. The most recent changes in the B. pertussis population involved the emergence of ptxP3 and fim3-2 strains. Consistent with this, the earliest Dutch isolates carrying the ptxP3 and fim3-2 alleles are from 1988 and 1994, respectively [Reference van Gent25]. Similar dates were observed in the USA [Reference Schmidtke40].
Mutations in B. pertussis are associated with clonal sweeps
Mutations in fim3, prn, ptxA and ptxP were associated with clonal sweeps, suggesting they increased strain fitness or were associated with (as yet unidentified) mutations that did so [Reference van Gent25] (Fig. 4). Vaccine-type strains (allele types II and III), predominant before the introduction of vaccination, were replaced by novel strains. Two types of changes were observed in the B. pertussis population, antigenic divergence with vaccine strains and increased Ptx production. Notably, the emergence of ptxP3 strains coincided with increased notifications. An association between the emergence of ptxP3 strains and increased notifications has also been observed in Finland and Australia [Reference Mooi22, Reference Octavia38]. Concordant changes in allele frequencies and notifications were also observed in the USA [Reference Schmidtke40]. In the USA, however, the association between ptxP3 frequencies and notifications was less tight than in The Netherlands and increases in notifications most closely followed fim3-2 frequencies. The fim3-2 allele is only found in association with ptxP3, and is thus difficult to separate effects caused by the two alleles. However, in The Netherlands the increase in notifications most consistently followed the increase in ptxP3 frequencies. In the Netherlands, fim3-2 strains increased in frequency, from 4% in 1996 to 62% in 2002, after which these strains gradually decreased in frequency to 9% in 2007 [Reference van Gent25]. In this period of declining fim3-2 frequencies, notifications remained high, suggesting that fim3-2 has played only a minor role, if any, in the increase in notifications. The differences observed between the USA and The Netherlands may be due to the different vaccines used. In The Netherlands a WCV was used until 2005, while in the USA WCVs were replaced by ACVs in 1997 [Reference Schmidtke40]. The common factor is, however, the link between the emergence of a distinct B. pertussis lineage and the increase in notifications. Interestingly, prn2, ptxP3 and fim3-2 were first detected in, respectively, the early 1980s, 1988–1989, and 1994, in both the USA and The Netherlands, suggesting rapid global spread of strains carrying novel adaptive mutations.

Fig. 4 [colour online]. Temporal trends in strain frequencies and notifications in The Netherlands during 1949–2010. Strain frequencies are indicated by coloured lines. Strains were aggregated into allele types (ATs) defined by the combination of alleles for ptxP, ptxA, prn and fim3 as shown in the top of the graph. No distinction was made between strains with the prn2 and prn3 alleles. ATs are indicated by blocked Roman numerals and allele changes resulting in differences between ATs are indicated. ATs found in one or two periods only, with a frequency lower than 15%, are not shown. If necessary, years were combined to increase the number of analysed strains to at least six. Note that due to this, the x-axis is not proportional. Changes in the vaccination programme are indicated below the x-axis. From 1953 to 2005 a whole cell vaccine (WCV) was used. In 2002, a booster with an acellular vaccine (ACV) was introduced for 4-year-olds and in 2005 the WCV was replaced by an ACV for all age groups. (Adapted from van Gent et al. [Reference van Gent25].)
P3 strains: a globally emerged lineage
Phylogenetic studies showed that the ptxP3 strains isolated in the Americas, Asia, Australia and Europe formed a monophyletic branch which recently diverged from ptxP1 strains [Reference van Gent43]. First detected in the late 1980s, ptxP3 strains are now found worldwide and in several countries they have reached frequencies of more than 90%, essentially replacing the resident ptxP1 B. pertussis population [Reference Schouls21, Reference Mooi22, Reference Advani36, Reference Kallonen37, Reference Petersen39, Reference Schmidtke40]. This rapid global expansion of ptxP3 strains is remarkable. The ptxP3 strains produce more Ptx than the ptxP1 strains they replaced, providing a rationale for their emergence and spread. It has been well established that Ptx plays a central role in suppression of both the innate and acquired immune system [Reference Carbonetti45]. Thus, in primed hosts, increased Ptx production may delay an effective immune response, enhancing transmission and hence pathogen fitness. Increased Ptx production may also be beneficial for the pathogen because the host requires higher levels of antibodies against Ptx for toxin neutralization.
Ptx causes leukocytosis in humans by inhibiting regression of leukocytes from the vasculature, and high levels of leukocytosis are associated with an increased mortality rate in infants due to pulmonary hypertension [Reference Pierce, Klein and Peters46]. Thus, the invasion of ptxP3 strains may result in increased illness and death. Although there is some evidence that ptxP3 strains are more virulent [Reference Mooi22, Reference Advani47], further research is needed to resolve this issue. For this purpose, comparing the clinical picture and outcome between ptxP3- and ptxP1-infected individuals would be valuable.
The effect of ACVs on B. pertussis populations
The changes in allele frequencies observed in B. pertussis populations, predate the introduction of ACVs and were thus presumably primarily driven by vaccination with WCVs. ACVs were introduced between 1994 and 2005 in a number of countries and may exert selective pressures that are qualitatively and quantitatively different from WCVs. WCVs induce a Th1 cytokine profile while the response after ACV vaccination shows a mixed Th1/Th2 profile [Reference Higgs15]. Further, WCVs induce a broad immune response, with relatively low titres against individual antigens, while ACVs induce an immune response against only a few antigens, but with higher titres [Reference Edwards48]. Therefore, the introduction of ACVs may eventually result in new adaptations in the B. pertussis population. Indeed, after the introduction of ACVs in France, Japan, Finland and The Netherlands, strains have been found that do not express FHA, Ptx or Prn, three components of the currently used ACVs [Reference Bouchez49–Reference Barkoff51] (our unpublished data). In France and Japan, strains that do not express Prn have reached frequencies of 14% and 27%, respectively. As yet, the effects of the loss of Prn production on vaccine efficacy and strain fitness have not been quantified.
Strain variation affects colonization of naive and immune mice
Functional studies in animal models are important to substantiate epidemiological associations. Several groups have studied the effect of B. pertussis strain variation on vaccine efficacy in mice. Ideally, such studies need to reflect the conditions in human populations, where newly emerged strains are most successful in individuals in which immunity has waned. Four studies revealed an effect of strain variation on vaccine efficacy [Reference King52–Reference Komatsu55]. Particularly elegant was the study by Komatsu and co-workers [Reference Komatsu55]. These authors constructed isogenic strains differing only in the ptxA and/or prn alleles and showed that mismatches with the vaccine strain in both alleles was required to reduce vaccine efficacy in a mouse model. The effect of the ptxP3 allele on vaccine efficacy has not yet been studied in mice. However, studies in naive mice can also shed light on the relevance of strain variation. When we tested a large number of clinical isolates in naive mice, only variation in Prn and ptxP were found to significantly affect colonization [Reference van Gent56]. Variation in Prn is mainly found in region R1, which is located proximal to the RGD motif implicated in host-cell attachment [Reference Leininger57]. Thus variation in R1 may affect both immune recognition and binding to host cells, explaining why polymorphism in Prn was found to affect colonization of both naive and immune mice [Reference King52, Reference van Gent56]. In naive mice, Prn1 strains were more proficient colonizers than Prn2 and Pn3 strains, although only the difference with Prn3 was statistically significant. In immune mice, however, Prn2 were the best colonizers. These observations are consistent with the predominance of Prn1 and Prn2 strains in unvaccinated and vaccinated populations, respectively.
DISCUSSION
Studies of B. pertussis populations suggest that, even in the context of complex bacterial genomes, small mutations in single genes can have a significant effect on strain fitness, resulting in clonal sweeps within a period of 6–20 years [Reference van Gent25]. This implies that B. pertussis is a well-adapted pathogen which requires mainly genetic fine-tuning to persist and resurge in the face of vaccination. Perhaps this is because B. pertussis contains a large gene repertoire focused on manipulating and suppressing host defences [Reference de Gouw13]. As suggested by the emergence of ptxP3 strains, small genetic changes in bacterial pathogens may be of significant relevance for public health.
Changes in the B. pertussis population, similar to those in The Netherlands, have been observed in many countries. Yet they have not always been followed by (large) increases in notifications. It is unclear whether these discrepancies are due to differences in surveillance methods [Reference He58] or differences in population immunity. By standardization of surveillance it should be possible to distinguish between the two possibilities and to select vaccines and vaccination strategies that are most effective.
Pathogen adaptations reveal weak spots in the bacterial defence and hence point to ways to improve vaccination. For example, memory induction and the effectiveness of antibodies may be improved by updating vaccines to include protein variants that predominate in current populations. The emergence and global spread of strains with increased Ptx production underline the central role Ptx plays in the ecology of pertussis. Thus, persistence of sufficient high levels of Ptx neutralizing antibodies may be the clue to resolving the pertussis problem. In light of this, the use of boosters, with low Ptx content, for infants and adults should be carefully (re)considered as fewer side-effects, due to the reduced antigen content, should be balanced against increased infection rates if the duration of protection is affected. The quality and persistence of Ptx antibodies can be improved by replacing chemically detoxified Ptx with genetically detoxified Ptx. Genetically detoxified Ptx is more immunogenic than chemically detoxified Ptx and also induces Ptx neutralizing antibodies more efficiently [Reference Greco59]. While changing the composition of pertussis vaccines may be a long-term project, morbidity and mortality in infants can be reduced significantly in the short term by maternal immunization or cocooning strategies [Reference Mooi and de Greeff60, Reference de Greeff61]. In fact, maternal immunization is now recommended in the USA and UK [62, 63]. The pertussis epidemics in the last 3 years may give us some respite as population immunity has been boosted by natural infection. However, this should not give us a (false) sense of security as there is no evidence that the increase of pertussis infections in adolescents and adults is waning.
Changes observed in B. pertussis populations are predicted to affect the duration of protection (and thus the waning of immunity). Antigenic divergence with vaccine strains will affect both memory recall and the efficacy of antibodies. Further, higher levels of Ptx, may increase suppression of the innate and acquired immune system, allowing B. pertussis strains to outpace antibody recall in hosts in whom immunity has waned. The solution to the pertussis problem requires a comprehensive approach focused on the characteristics of the vaccines, the B. pertussis populations and the interaction between the two.
ACKNOWLEDGEMENTS
This work would not have been possible without the contributions of Han van der Heide, Kees Heuvelman, Marjolein van Gent, Marieke Bart, Anne Zeddeman and Sabine de Greeff. We are also grateful to the Medical Microbiology Laboratories which provided strains for typing.
DECLARATION OF INTEREST
None.







