INTRODUCTION
Vitamin D is produced in the skin when sunlight is absorbed. Thus, vitamin D levels, or serum 25-hydroxyvitamin D (25-OHD), fluctuate seasonally. 25-OHD levels are low during winter in northern latitudes because of decreased amounts of sunlight. A conventional diet usually does not provide adequate amounts of vitamin D. Vitamin D insufficiency results in a number of skeletal and extra-skeletal complications when serum 25-OHD concentrations are <75 nmol/l. It has been associated with reduced calcium absorption, osteoporosis and increased fracture risk [Reference Heaney1]. It has also been associated with decreased muscle strength [Reference Visser, Deeg and Lips2], breast cancer [Reference Bertone-Johnson3, Reference Lappe4], colon cancer [Reference Freedman, Dosemeci and McGlynn5], cardiovascular disease [Reference Schmidt-Gayk6], and autoimmune disorders such as rheumatoid arthritis [Reference Cutolo7] and systemic lupus erythematosus [Reference Cutolo and Otsa8]. In vitro and in vivo studies show a role for vitamin D as an important component of the innate immune system. The innate immune system provides front-line protection against infectious agents. Expression of vitamin D receptor was found in different cells of the myeloid and lymphoid lineage. The active form of vitamin D, 1,25-dihydroxyvitamin D (1,25-OH2D) increases the production of endogenous antibiotics called antimicrobial peptides (AMP) in human monocytes, neutrophils, and epithelial cells [Reference Wang9]. AMPs such as defensin and cathelicidin have a broad range of actions against microorganisms, including bacteria, fungi and viruses. Defensins can block viral infection by directly acting on the virion or by affecting the target cell and thereby indirectly interfering with viral infection [Reference Klotman and Chang10]. One of the defensins called retrocyclin-2 inhibits influenza virus infection by blocking membrane fusion mediated by the viral haemagglutinin [Reference Leikina11].
Liu et al. showed that stimulation of toll-like receptors (TLR) 2/1 engages a vitamin D-dependent intracellular circuit that results in the expression of cathelicidin, enhancing the microbicidal capability of the monocyte [Reference Liu12]. Markedly, these authors also observed that sera from African American individuals, who are known to have substantially lower serum 25-OHD levels than whites [Reference Harris and Dawson-Hughes13] were inefficient in inducing genetic expression of cathelicidin. When these authors supplemented the sera with 25-OHD, cathelicidin levels increased to levels observed in monocytes collected from whites. This suggests that vitamin D insufficiency during winter may be the ‘seasonal stimulus’ that increases susceptibility to infections, particularly viral respiratory infections including influenza virus, as first suggested by Hope-Simpson in 1987 [Reference Hope-Simpson and Golubev14] and 1992 [Reference Hope-Simpson15].
It is estimated that 72% of adults experience at least one URI per year and those adults experience an average of 2·5 URIs per year [Reference Fendrick16]. Every year 5–20% of the USA population get influenza [17]. Vitamin D may also play a role in reducing the severity of URI symptoms by regulating specific cytokines that participate in the host inflammatory response. 1,25-OH2D has been shown to inhibit mononuclear and T lymphocyte cell proliferation by decreasing the production of IL-1β, IL-2, IL-6, interferon γ (IFN-γ), and TNF-α [Reference Lemire18]. A randomized controlled trial by Schleithoff et al. showed that 50 μg/day (2000 IU/day) vitamin D3 reduced the inflammatory milieu in congestive heart failure patients [Reference Schleithoff19].
When reviewing the adverse events from our previous study of vitamin D3 supplementation in post-menopausal African American women [Reference Aloia20] we noticed a significant difference in the reported incidence of URI symptoms between the vitamin D and placebo groups. In this 3-year randomized controlled trial, the subjects received either placebo or 20 μg/day vitamin D3. After 2 years, the vitamin D3 dose was increased to 50 μg/day in the active group. There were 39 reports of URI symptoms: 30 in the placebo group vs. 9 in the vitamin D group (P<0·0002). When we examined the seasonality of the symptoms, we found that the placebo group had URI symptoms mostly in winter. The vitamin D group had symptoms throughout the year while on 20 μg/d, whereas only one subject had a URI while on 50 μg/d. This finding led us to conclude that higher doses of vitamin D supplementation may protect against symptomatic URIs.
Current recommendations for vitamin D intake are based on the amounts required to sustain optimal skeletal health. Schleithoff et al.'s study and our previous study suggest that optimal function of the innate immune system might require higher doses of vitamin D. The current recommended intake of vitamin D for adults is 400 IU/day (10 μg/day). In this study we administered 50 μg/day (2000 IU) vitamin D3 which is the tolerable upper intake level of vitamin D for children and adults set by the Food and Nutrition Board of the Institute of Medicine [21]. Experts in the USA believe that higher intakes of vitamin D are necessary and that these higher intakes are safe [Reference Heaney22, Reference Vieth23].
The aim of this study was to evaluate whether vitamin D3 supplementation of 50 μg/day during the winter season prevents symptomatic URIs in adults, and whether vitamin D3 supplementation decreases the severity and duration of URI symptoms.
SUBJECTS AND METHODS
Subjects
Study participants were recruited from the Long Island, New York community (latitude, 40·7° N) between December 2006 and March 2007 (Fig. 1). Volunteers were recruited from local newspaper advertisements, mailing of brochures to community residents, and flyers posted at Winthrop University Hospital medical offices. Patients were eligible for the study if they met the following criteria: ambulatory adult age 18–80 years and stable medical condition with no change in medications for 6 months prior to study entry. Exclusion criteria included morbid obesity (body mass index >35 kg/m2); current tobacco use; history of hypercalcaemia, nephrolithiasis or sarcoidosis; pregnancy; recent hospitalization; current liver or kidney disorders, malignancy and malabsorption; and use of immunosuppressants or medications that interfere with vitamin D metabolism such as phenytoin and carbamazepine. Race determination was by self-declaration. All participants provided written informed consent and the trial was approved by the institutional review board of Winthrop University Hospital.

Fig. 1. Flowchart of study participants.
Study design
This study was a 3-month prospective, randomized, double-blind, placebo-controlled trial of vitamin D3 supplementation in ambulatory adults. Recruitment began in December 2006 to March 2007 and the study was completed in June 2007. The participants were randomly assigned using a computer-generated randomization sequence to receive either 50 μg/d vitamin D3 or matching placebo. Each subject was sequentially assigned a number upon study entry and the investigators dispensed the corresponding sequentially numbered container of study medication to the subject. All participants and investigators were blinded throughout the study except for the research pharmacist and the statistician. Neither the statistician nor the research pharmacist had any contact with study participants. Dietary calcium and vitamin D intake was assessed using a food frequency questionnaire. Eligible subjects underwent a baseline medical history, height and weight measurements, and blood tests. Subjects were seen at 6 weeks and 12 weeks post-randomization. Blood was collected again at the 12-week visit.
URI assessment
A bi-weekly questionnaire (available in the online version of the paper) modified from established, validated instruments [Reference Chubak24, Reference Nieman25] was used to record the incidence of URI symptoms in the subjects. The questionnaire inquired about symptoms of URI, duration and severity of symptoms, sick contacts, medication use, sick leave due to illness, and doctor visits. The questionnaire was available online for subjects to complete. Subjects were emailed bi-weekly reminders to complete the questionnaire or they were mailed questionnaires bi-weekly if they preferred to receive them by mail. Subjects were called by phone if they did not answer a questionnaire after 4 weeks. URI was defined as the presence of two or more URI symptoms (fever, cough, productive sputum or change in sputum color and quantity, muscle aches, nausea or vomiting) and the absence of allergy symptoms (clear nasal discharge, watery eyes, and itchy nose).
Laboratory tests
Serum 25-OHD was measured by a radio-receptor assay from DiaSiorin Inc. (USA). The intra-assay variability in our laboratory is 4·1% and inter-assay variability is 7·0%. Our laboratory participates in the international Vitamin D External Quality Assessment Program (http://www.deqas.org). Serum parathyroid hormone (PTH) was measured by the Immulite 2000 Analyzer for the quantitative measurement of intact PTH (Diagnostic Products Corporation, CA). Vitamin D3 content was analysed in an independent laboratory (Vitamin D, Skin, and Bone Research Laboratory, Department of Medicine, Boston University School of Medicine, Boston, MA, USA).
Statistical analysis
To assess the effect of vitamin D supplementation on the incidence of URI, elementary χ2 test, correlation, and t tests (both independent and paired) were used. Severity and duration of URIs were measured both across the overall sample of URI reports as well as stratified by patient. Mean severity and duration was calculated first with missing values set to zero (i.e. treating those who do not report a URI as having zero severity and duration) and then again with missing values ignored (i.e. calculating mean severity and duration only in those who actually report a URI). In the main analysis all data were used, but secondary analyses ignored the data for the first 4 weeks because it takes about 3 months for vitamin D levels to approach steady state [Reference Vieth, Chan and MacFarlane26]. As results under all combinations of the above scenarios did not differ, only results calculated within subjects who actually report a URI using all available data are reported. A mixed-model repeated-measures analysis of variance measured continuous change (e.g. severity of illness) over time in the vitamin D group compared to the placebo group. An ‘exchangeable’ covariance structure was used in the repeated-measures analysis, i.e. the working correlation structure assumed a constant correlation between all time points. The General Linear Model (GenMod) was used to analyse the incidence of URIs in repeated measures on the same patient. Means were expressed as 95% confidence intervals when appropriate. P<0·05 was the nominal definition of statistical significance. All analyses were carried out using SAS version 9·1 (SAS Institute Inc., USA).
RESULTS
Baseline characteristics
The baseline characteristics and laboratory values of the study population are summarized in Table 1. There were no significant differences between the active and placebo patients at baseline. The baseline 25-OHD levels ranged from 16 to 156 nmol/l with a mean level of 63·7±28·7 nmol/l in the study population. At baseline, 23% of the active patients exceeded 75 nmol/l.
Table 1. Baseline characteristics of study participants. There were no significant differences between the study groups at baseline

BMI, Body mass index; PTH, parathyroid hormone; COPD, chronic obstructive pulmonary disease.
Adherence
Adherence (defined as the ratio of the number of pills consumed to the number of days in the study) ranged from 59% to 100%. Mean compliance was 94±9%. Adherence did not significantly differ between the active and placebo groups. Fourteen patients discontinued the study (Fig. 1). Twelve of the 14 patients discontinued by week 6 of the study. The other two discontinued at week 8 of the study.
Incidence of URIs
A total of 751 questionnaires were returned by the participants. Each participant returned an average of five questionnaires during the 12-week study. There was no difference in the incidence of URIs between the vitamin D and placebo groups. There were 48 URIs out of 388 reports in the vitamin D group and 50 URIs out of 363 reports in the placebo group. This difference of 1·4% in favour of vitamin D (95% CI −2·4 to 3·4) was not statistically different (P=0·56). There was also no observed difference in the number of subjects who had at least one URI between the vitamin D group (28 patients, 36%) and the placebo group (29 patients, 41%). The 5% difference in URI incidence rate (95% CI −12 to 20) in favour of the vitamin D group was not statistically different (P=0·74). A GenMod analysis, accounting for the clustering of URIs within patients, did not reveal any differences in respect of URI incidence in the two groups (P=0·61). We also analysed the results after excluding the first 4 (and 2 and 6) weeks of questionnaires, considering that it takes 3 months for vitamin D levels to increase, and found no difference in the incidence of URIs between the treatment and placebo groups.
Severity and duration of URIs
There was no difference in the duration of URIs between the vitamin D and placebo groups (95% CI for the difference in duration −1·8 to 2·1) (Table 2). There was also no difference in the severity of URIs between the two groups. Participants rated the severity of their symptoms on a 5-point scale (1=healthy, 5=‘very ill’). There was no statistically significant linear relationship (correlation coefficient) between 25-OHD levels and the number of URIs, the number of symptoms, the severity of symptoms or the duration of the URI. There was no difference in the frequency of URI symptoms between active and placebo patients except for muscle aches: 2% of active patients complained of muscle aches compared to 6% of placebo patients (P<0·01).
Table 2. Characteristics of URIs by group
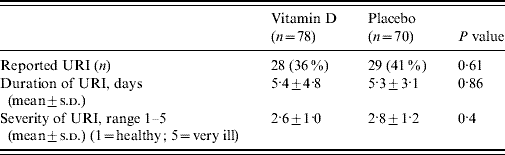
URI, upper respiratory tract infection.
Laboratory values
Blood samples were collected from a large subset of patients (150/162 patients, 92·6%). 25-OHD levels (expressed as nmol/l) increased in the vitamin D group from a mean of 64·3±25·4 to 88·5±23·2. This difference of 24 nmol/l (95% CI 21·7–30·8) was statistically significant compared to the change in the placebo group which had a mean change of −2·1 nmol/l (95% CI −7·1 to 2·8, P<0·0001). At the end of the study, 73% of the active patients exceeded 75 nmol/l. Serum calcium levels remained the same in both groups (9·3 mg/dl at the beginning and end of study).
Adverse events
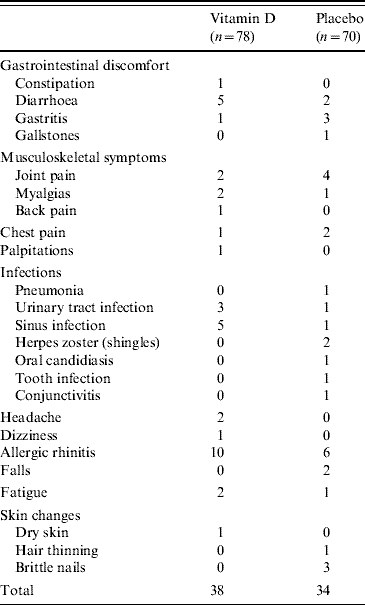
A total of 72 adverse events were reported in the study over 3 months, 38 in the vitamin D group and 34 in the placebo group (P=0·99) (Table 3). There was no significant difference in the adverse events between the study groups. There were three serious adverse events, one in the vitamin D group and two in the placebo group. One patient in the vitamin D group had chest pain requiring hospitalization with no evidence of myocardial infarction. One patient in the placebo group was hospitalized with high fever and chills with no evidence of bacterial infection. Another patient in the placebo group broke an ankle after a fall. None of the serious adverse events were considered to be related to the study medication. There were no episodes of nephrolithiasis or hypercalcaemia.
Table 3. Adverse events
DISCUSSION
This is the first randomized, double-blind, placebo-controlled trial of vitamin D3 supplementation for URI prevention that we are aware of. We found no benefit of vitamin D3 supplementation in decreasing the incidence or severity of URIs during the winter months. Our results differed from Laaksi et al. who found that low 25-OHD levels in young Finnish men were associated with more absences due to respiratory infection (incidence rate ratio 1·63, 95% CI 1·15–2·24) [Reference Laaksi27]. A sub-study analysis of the RECORD (Randomised Evaluation of Calcium and/OR vitamin D) trial showed a trend towards lower rates of infection in the vitamin D group, but the results were not statistically significant [Reference Avenell28]. Of note, the RECORD trial used a lower dose of vitamin D3, i.e. 20 μg/day.
Mild URIs occurring during the winter months are most often due to viruses, e.g. respiratory syncytial virus (RSV). During late winter/early spring, the influenza season begins. Influenza runs the clinical spectrum of mild disease, resembling RSV, to fulminant/fatal influenza pneumonia. Influenza A is more severe than influenza B and affects adults more commonly than children. Mild influenza A is clinically indistinguishable from influenza B and can only be differentiated by specific diagnostic testing serologically or by antibody testing and sputum or in respiratory secretion specimens. Influenza-like illness (ILI) is a symptomatic definition and can include both RSV and influenza infections, as well as other viral infections that cause fever, nausea, myalgia, and/or cough. The 2006–2007 influenza season in Long Island, NY matched patterns in the mid-Atlantic region where levels of influenza activity increased during January, peaked in early March, and decreased in May [29, 30].
There are several reasons why vitamin D3 supplementation may have been ineffective at preventing URIs in this study. First, the subjects started vitamin D3 supplementation during the wintertime and not beforehand. It takes about 3 months for 25-OHD levels to reach a steady state with supplementation [Reference Vieth, Chan and MacFarlane26, Reference Barger-Lux31]. Because it takes a significant amount of time to build up vitamin D stores, the effect of vitamin D supplementation lagged behind the cold and influenza season. Vitamin D3 supplementation may be more effective in preventing URIs if started during autumn when sunlight begins to decrease. Another reason why we may not have observed a benefit is that 50 μg/day may not be enough to stimulate innate immunity, although in our previous study this dose led to decreased reports of wintertime URIs in African American women. African Americans are known to have lower vitamin D levels because of darker skin pigmentation [Reference Harris and Dawson-Hughes13]. The subjects in our previous study started with a lower baseline 25-OHD level of 46·9±20·6 nmol/l (95% CI 43·9–50·39) when compared to the baseline level of the subjects in this study (63·7±28·7 nmol/l) but they reached similar 25-OHD levels of 86·9±27·0 nmol/l (95% CI 80·1–94·1, P<0·001) after being on 50 μg vitamin D3 daily for 3 months [Reference Aloia20]. Lower vitamin D levels, as shown in Liu et al.'s study [Reference Liu12], were associated with decreased cathelicidin expression, thus vitamin D supplementation may have a greater benefit in subjects with lower baseline vitamin D levels. In this study, only 4% of the study participants were African American. The effect of vitamin D3 supplementation on URI prevention may not be as pronounced in this study population consisting mostly of Caucasians because the baseline 25-OHD levels were higher.
The strengths of the present study include the high compliance rate of medication intake (94%) and low dropout rate. We also used an objective definition of URI to include two or more symptoms in the absence of allergy symptoms. We had high cooperation in return of surveys because most of the subjects completed the questionnaires online. Thus, we had real-time collection of data which decreases recall bias since the time interval between each survey was only 2 weeks. The mean 25-OHD level of the subjects at baseline (63·7±28·7 nmol/l) was in accord with the mean 25-OHD level in NHANES III (64·8 nmol/l) measured during winter [Reference Looker32]. Our laboratory participates in the international Vitamin D External Quality Assessment Program which ensures the analytical reliability of 25-OHD assays. Vitamin D content in the tablets was verified by an independent laboratory. We also used vitamin D3 instead of vitamin D2 which some believe increases 25-hydroxyvitamin D levels more effectively than vitamin D2 [Reference Trang33, Reference Houghton and Vieth34].
We recognize the following limitations of this study. First, the subjects were not on vitamin D before the influenza season began. As mentioned previously, the effect of vitamin D supplementation on the immune system may be more evident with higher vitamin D levels achieved at the beginning of the influenza season. Second, the mean 25-OHD level at the beginning of the study was not that low and was similar to the level seen in NHANES III. Seventy-three per cent of patients in the vitamin D group achieved 25-OHD levels >75 nmol/l but the difference between baseline and end-of-study levels may not be enough to confer a benefit. Third, the study was powered initially to detect a 25% difference in the incidence of URIs in the placebo group vs. the vitamin D group. The literature suggested that 72% of adults have at least one URI every year. To be conservative we assumed that the incidence of URIs in the placebo vs. vitamin D groups would be 55% vs. 30%, respectively, and calculated that a sample of 85 patients in each group would allow us to observe statistical differences with 92% power. We assumed a 12-week attrition rate of 15%, so that the recruitment target was 100 patients in the active and placebo groups. Although we did not meet our enrolment goals, we still had 80% power to detect a 23% difference in the incidence of URIs between the vitamin D and placebo patients. However, because the two groups were actually very close, the more relevant measure is the 95% confidence interval for the difference in incidence rates. That interval is −12% to 20%. One can interpret from our results (at 50 μg/day) that we are 95% confident that vitamin D did not decrease the incidence of URIs by >20%. If a smaller difference between active and placebo is chosen to be detected (i.e. <20%), a study with a larger sample size and/or a greater dose would be required. Another factor that could have diminished the difference between the vitamin D and placebo groups is the high influenza vaccination rate in both groups (56·4% of subjects in vitamin D group, 64·3% in placebo group). This indicates a relatively health-conscious population. Vaccination could have decreased the overall incidence of symptomatic URI, thus reducing the power.
Although the present study did not show a benefit of vitamin D3 supplementation in preventing viral URIs, there is accumulating evidence that vitamin D does significantly alter the immune system. Because this study was conducted in ambulatory patients, patients with mild URIs probably had colds due to various viruses. The host defence that prevents intracellular viral replication is cell-mediated immunity (CMI) by T lymphocytes. Vitamin D may enhance CMI which may be effective in severe/hospitalized cases of influenza. Since months are required for vitamin D levels to achieve peak/steady-state concentrations, further studies should be done giving vitamin D during autumn, before the influenza season and specifically study severe/hospitalized patients with influenza to determine if vitamin D has a salutary effect on this patient subset.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
ACKNOWLEDGEMENTS
This research was partially funded by the Empire Clinical Research Investigator Program (ECRIP). We thank Tatiana Baron, D.O., Cindy Bredefeld, D.O., Lawrence Eisenstein, M.D., Sara Nausheen, M.D. and Uzma Syed, D.O. for their clinical care and data gathering. We thank Jane Greensher for her expertise as the Nurse Coordinator, and Marty Feuerman for contributing to the data and statistical analyses.
DECLARATION OF INTEREST
None.





