INTRODUCTION
Streptococcus pneumoniae is one of the major pathogens causing community-acquired pneumonia, meningitis, sinusitis, acute otitis media, and bacteraemia. Over the past three decades antibiotic-resistant strains have emerged and spread rapidly in both industrialized and developing countries [Reference Adam1–Reference Whitney3]. Despite the increased prevalence of antibiotic-resistant S. pneumoniae, the clinical impact of resistant strains remains unclear. Whereas therapeutic failure due to penicillin [Reference Metlay4–Reference Sloas6], macrolide [Reference Kelley7–Reference Van Kerkhoven9], and fluoroquinolone [Reference Davidson10] resistance has been reported in cases of meningitis, otitis media, and pneumonia, the relationship between antibiotic resistance and treatment remains controversial. In particular, there is a paucity of data on outcomes in patients with invasive pneumococcal disease, although studies of invasive pneumococcal isolates have suggested that antibiotic resistance is not a risk factor for mortality [Reference Moroney11, Reference Yu12]. Moreover, there has been minimal research on the clinical features and outcomes of macrolide resistance in Asian countries, where the highest prevalence of resistance to macrolides, ranging from 33% to 92%, has been reported [Reference Song2, Reference Song13–Reference Song15]. We therefore conducted a retrospective observational cohort study to evaluate the risk factors for mortality in adult patients with pneumococcal bacteraemia and to assess the impact of antimicrobial resistance on clinical outcomes.
METHODS
Patients
We conducted a review of the clinical microbiological laboratory results and medical records of individuals diagnosed with pneumococcal bacteraemia from July 1996 to June 2006 at Seoul National University Hospital (Seoul, Republic of Korea), a 1600-bed tertiary-care university hospital and referral centre. Only the first bacteraemic episode for each patient was included in the clinical analysis.
In vitro susceptibility testing
S. pneumoniae strains were identified by Gram stain, optochin susceptibility and bile solubility test. Susceptibility to all the antimicrobials administered in pneumococcal infections was determined by E-test (AB Biodisk, Sweden) in accordance with the manufacturer's instructions, and on the basis of the Clinical and Laboratory Standards Institute (CLSI) breakpoints [16]. The isolates were tested for susceptibility to the following antibiotics: penicillin, amoxicillin/clavulanic acid, cefotaxime, ceftriaxone, chloramphenicol, clindamycin, erythromycin, imipenem, levofloxacin, rifampin, tetracycline, and trimethoprim-sulfamethoxazole. In addition, E-test for cefuroxime was performed for cefuroxime-treated patients in order to better evaluate the adequacy of the antibiotic. Clarithromycin and azithromycin were considered to be the same as erythromycin with regard to susceptibility. S. pneumoniae ATCC 49619 was used as a control. Penicillin minimum inhibitory concentration (MIC) was categorized as susceptible, intermediate or resistant according to revised 2008 CLSI guidelines [16] where the isolates are classified into three groups, i.e. meningitis with parenteral penicillin where MIC ⩽0·06 μg/ml is susceptible and MIC ⩾0·12 μg/ml is resistant; non-meningitis with parenteral penicillin, where MIC ⩽2, 4 and ⩾8 μg/ml are susceptible, intermediate and resistant, respectively; and oral penicillin, where MIC ⩽0·06, 0·12–1, and ⩾2 μg/ml are susceptible, intermediate, and resistant, respectively. Cefotaxime or ceftriaxone breakpoints for non-meningeal isolates are: susceptible, ⩽1 μg/ml; intermediate, 2 μg/ml; resistant, ⩾4 μg/ml. For meningitis, the breakpoints are: susceptible, MIC ⩽0·5 μg/ml; intermediate, 1 μg/ml; resistant, ⩾2 μg/ml. For the purposes of the study, we combined the isolates with intermediate susceptibility and resistance to antibiotics as antibiotic-non-susceptible strains.
Study design and data collection
A retrospective cohort study was conducted to evaluate the risk factors for mortality and to determine the impact of antimicrobial resistance on clinical outcomes. We reviewed the medical records of patients. Data collected included; age, sex, underlying diseases, laboratory investigations, chest radiographs or computerized tomographs at presentation, source of bacteraemia, severity of illness as calculated by the Acute Physiology and Chronic Health Evaluation (APACHE) II score [Reference Knaus17], antimicrobial therapy regimen, and times of administration of antibiotics and blood culture sample acquisition. The presence of the following comorbid conditions was also documented; neutropenia, presentation with septic shock, care in the intensive-care unit (ICU), receipt of chemotherapy within 30 days before the onset of bacteraemia, transplantation within 1 year before the onset of bacteraemia, corticosteroid use, post-operative status, and invasive procedure conducted during the 72 h before the onset of bacteraemia. Because this study was retrospective, the patients' physicians, not researchers, had chosen the antimicrobial therapy regimens. Thirty-day mortality was the main outcome measurement. Secondary outcome measures were 7-day mortality, time to defervescence, admission to ICU, septic shock, total duration of antibiotic therapy, and length of hospital stay. This study was approved by the Seoul National University Hospital Institutional Review Board.
Definitions
S. pneumoniae bacteraemia was defined as at least one positive blood culture, along with clinical features compatible with systemic inflammatory response syndrome. Bacteraemia was categorized as polymicrobial if additional microorganisms were recovered from blood cultures. Nosocomial infection was defined as an infection that occurred >48 h after admission to the hospital; an infection that occurred <48 h after admission to the hospital but in patients who had been hospitalized in the previous 2 weeks; and infection that occurred <48 h after admission to the hospital in patients who had been transferred from another hospital or nursing home. Neutropenia was defined as an absolute neutrophil counts <500/mm2. Septic shock was defined as sepsis associated with evidence of organ hypoperfusion and a systolic blood pressure <90 or >30 mmHg less than the baseline or a requirement for the use of vasopressors to maintain the blood pressure [Reference Dahmash, Chowdhury and Fayed18]. Antimicrobial therapy was considered appropriate if the treatment regimen during the first 48 h of treatment included at least one antibiotic to which the isolate was susceptible and if the dose and route of administration conformed to current medical standards [Reference Harbarth and Garbino19].
Data analysis
Student's t test was used to compare continuous variables, and χ2 or Fisher's exact test was used to compare categorical variables. To identify the independent risk factors for resistance and mortality, a multiple logistic regression model was used to control for the effects of confounding variables. The time to defervescence was calculated by Kaplan–Meier analysis. All P values were two-tailed, and P<0·05 was considered statistically significant, SPSS software, version 17.0 (SPSS Inc., USA), was used for the analysis.
RESULTS
Patient characteristics (Table 1)
One hundred and fifty consecutive patients with pneumococcal bacteraemia were enrolled in the study. The median age of the patients at diagnosis was 59 years, ranging from 23 to 85 years, and 103 (68·7%) patients were male. The most common underlying disease was solid organ tumour (n=56, 37·3%), and the most common primary site of infection was the lung (n=76, 50·7%). Of the 150 patients with pneumococcal bacteraemia, 122 (81·3%) had penicillin-susceptible (Pen-S) strains and 28 (18·7%) penicillin-non-susceptible (Pen-NS) strains; 43 (28·7%) had erythromycin-susceptible (EM-S) strains and 107 (71·3%) erythromycin-non-susceptible (EM-NS) strains. There were no significant differences in age, sex, origin of infection and site of infection between the two groups. Moreover, vital signs and laboratory values (serum sodium, creatinine, haematocrit, white blood cell count) at presentation did not differ in the two groups (data not shown). On the other hand, the mean APACHE II score was higher in the EM-NS group than in the EM-S group (17·99±7·83 vs. 14·42±6·44, P=0·01).
Table 1. Demographic and clinical characteristics of 150 patients with pneumococcal bacteraemia
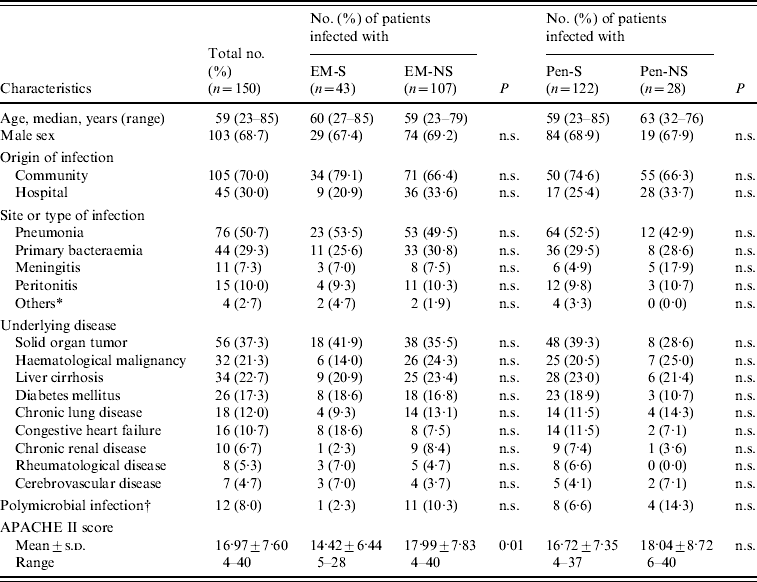
EM-NS, Erythromycin-non-susceptible; EM-S, erythromycin-susceptible; Pen-NS, penicillin-non-susceptible; Pen-S, penicillin-susceptible; n.s., not significant (P>0·05); s.d., standard deviation; APACHE, Acute Physiology and Chronic Health Evaluation.
Comparison based on χ2 or Fisher's exact test (when expected counts were <5 per cell).
* Pyogenic spondylitis (2), acute otitis media (1), cellulitis (1).
† Associated microorganisms were Enterobacter cloacae (1), Staphylococcus epidermidis (1), Staphylococcus aureus (2), Serratia marcescens (1), viridans streptococcus (1), Escherichia coli (2), Acinetobacter baumannii (1), Bacillus spp. (1), Morganella morganii (1), Pseudomonas aeruginosa (1).
Antimicrobial susceptibilities (Table 2)
Of the 150 isolates of S. pneumoniae, 107 (71·3%) were not susceptible to erythromycin, with very high levels of resistance to erythromycin (MIC range 0·016 to >256 μg/ml, MIC50>256 μg/ml, MIC90>256 μg/ml). On the other hand, 22 (14·7%) had intermediate susceptibility to penicillin, and six (4·0%) were resistant to penicillin (MIC range 0·006–16 μg/ml, MIC50 0·25 μg/ml, MIC90 3 μg/ml). Non-susceptibility rates to amoxicillin-clavulanate, cefotaxime, ceftriaxone, and levofloxacin were 3·3%, 12·0%, 5·4%, and 2·0%, respectively. The pattern of resistance to erythromycin did not change significantly over the 11-year study period.
Table 2. In vitro activity of 12 antimicrobial agents against 150 isolates of Streptococcus pneumoniae collected at Seoul National University Hospital from 1996 to 2006
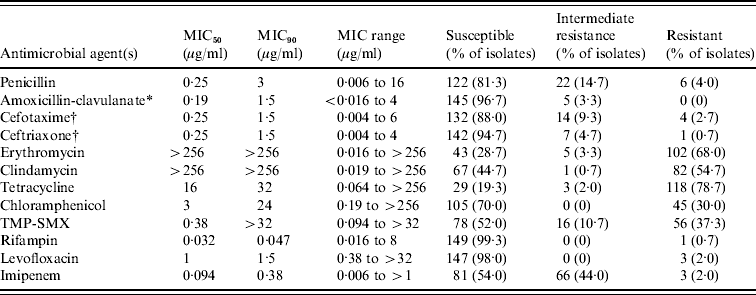
MIC, Minimum inhibitory concentration; TMP-SMX, trimethoprim-sulfamethoxazole.
* The ratio of the concentrations of amoxicillin and clavulanate was 2:1, and the data reflect the amoxicillin component.
† Using two separate interpretive breakpoints for meningeal and non-meningeal S. pneumoniae isolates to define cefotaxime and ceftriaxone resistance; MICs of ⩾2 and ⩾4 μg/ml, respectively.
Clinical outcomes and risk factors for mortality (Table 3 and 4)
The 30-day mortality rate for patients with pneumococcal bacteraemia was 26·7% (40/150 patients died). The overall 30-day mortality rate was 27·0% (33/121) in the Pen-S group and 25·0% (7/28) in the Pen-NS group; 20·9% (9/43) in the EM-S group and 29·0% (31/107) in the EM-NS group, both of which differences were not statistically significant. Further, no difference was observed in 7-day mortality rate between the two groups. There were no significant differences in septic shock, time to defervescence, total duration of antibiotic therapy, and length of stay, except that patients infected with Pen-NS strains were more likely to be admitted to an ICU than those infected with Pen-S strains (35·7% vs. 14·8%, P=0·02).
Table 3. Clinical outcomes of 150 patients with pneumococcal bacteraemia
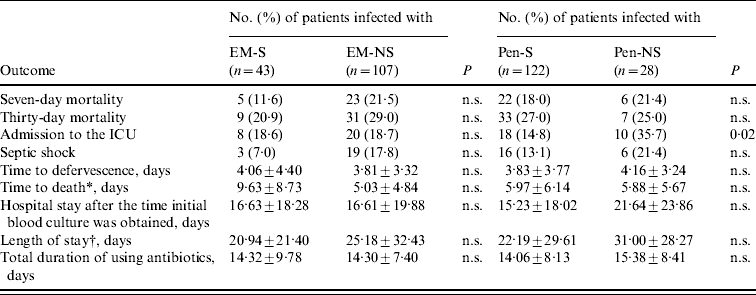
EM-NS, Erythromycin-nonsusceptible; EM-S, erythromycin-susceptible. Pen-NS, penicillin-nonsusceptible; Pen-S, penicillin-susceptible; n.s., not significant (P>0·05).
* Only in patients who died within 30 days of the initial drawing of blood for culture (n=40).
† Only in patients who survived (n=110).
Table 4. Factors associated with 30-day mortality in 150 patients with pneumococcal bacteraemia
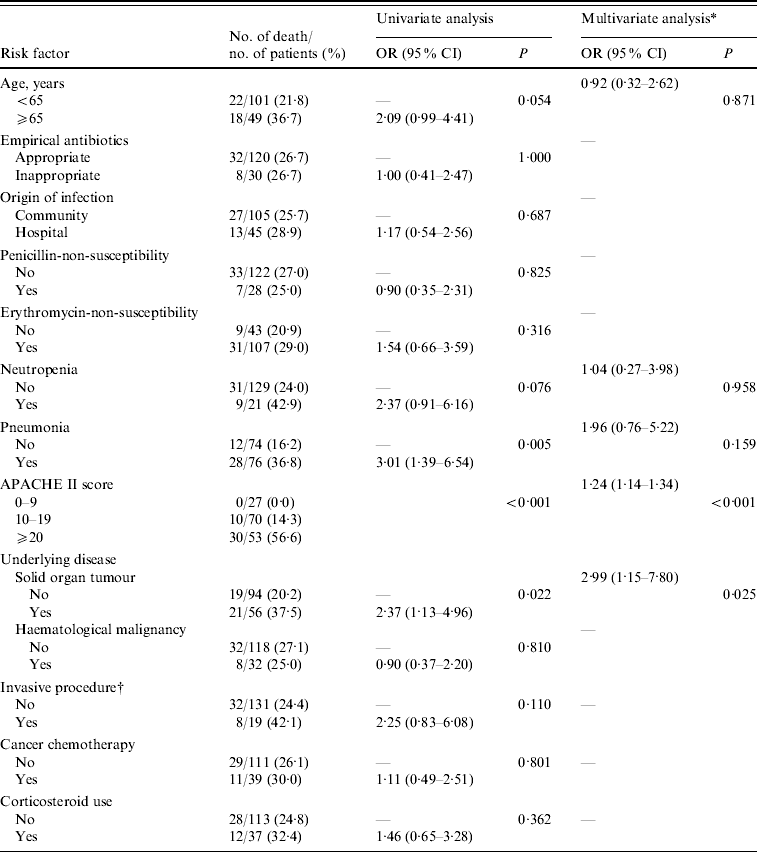
OR, Odds ratio; CI, confidence interval.
* Variables with P<0·1 in the univariate analysis were included in the multivariate analysis.
† Gastroscopy with or without sclerotherapy, endoscopic variceal ligation, insertion of a Sengstaken–Blakemore tube, colonoscopy, tracheal intubation, endoscopic retrograde cholangiopancreatography, and insertion of a percutaneous transhepatic biliary drainage catheter.
Of the 150 patients with pneumococcal bacteraemia, all but five patients were treated with antimicrobial agents. Forty-one patients (27·3%) received monotherapy, while 104 patients (69·3%) were treated with combination antimicrobials. There were no differences in the number and type of antimicrobial agents between the Pen-S group vs. the Pen-NS group and the EM-S group vs. the EM-NS group. To evaluate the influence of appropriate antibiotic therapy on mortality, we performed a subgroup analysis categorized by the adequacy of antibiotic therapy. The 30-day mortality rate in the appropriate antibiotic treatment group was 26·7% (32/120 patients), the same as in the inappropriate antibiotic treatment group (26·7%, 8/30 patients). When patients were divided by type of infection, underlying disease, and origin of infection, no significant differences in mortality were found between the appropriate and inappropriate antibiotic therapy groups (data not shown).
From the univariate analysis (Table 4), variables associated with 30-day mortality included pneumonia, solid organ tumour, and elevated APACHE II score (P<0·05 in each case). No significant differences were observed in the mortality associated with either Pen-NS (P>0·05) or EM-NS (P>0·05). Multivariate analysis using a logistic regression model, and including the variables associated with mortality by univariate analysis (P<0·1), showed that elevated APACHE II score [odds ratio (OR), 1·24, 95% confidence interval (CI), 1·14–1·34, P<0·001) and solid organ tumour (OR 2·99, 95% CI 1·15–7·80, P=0·025) were independent risk factors for mortality.
Time to defervescence of patients with pneumococcal bacteraemia
To assess the impact of antibiotic resistance on clinical response as measured by time to defervescence, we compared the patients infected with Pen-S strains with those infected with Pen-NS strains and patients infected with EM-S strains with those infected with EM-NS strains, respectively. Time to defervescence was not different in groups of patients infected with susceptible strains vs. non-susceptible strains. However, a statistical analysis of 76 separate patients with bacteraemic pneumococcal pneumonia (Fig. 1) revealed that the time to defervescence was longer in the EM-NS group than the EM-S group (5·45±4·39 vs. 2·93±2·56, P=0·03 by log rank test).

Fig. 1. Times to defervescence in 76 patients with bacteremic pneumococcal pneumonia. EM-NS S. pneumoniae tends to result in prolongation of fever (log rank=0·03). EM-NS, Erythromycin-non-susceptible; EM-S, erythromycin-susceptible.
DISCUSSION
In the present study we found that neither erythromycin nor penicillin resistance had a significant effect on clinical outcomes for patients with pneumococcal bacteraemia. However, among the patients with bacteraemic pneumococcal pneumonia, the time to defervescence in patients infected with EM-NS strains was longer than in patients with EM-S strains.
Despite the worldwide escalation in antimicrobial resistance over the past three decades, mortality rates for invasive pneumococcal disease have not increased [Reference Doern20, Reference Lynch and Zhanel21]. Treatment failures associated with penicillin resistance have been reported for meningitis [Reference Sloas6], otitis media [Reference Poole5], and pneumonia [Reference Metlay4], but the relationship between penicillin resistance and increased mortality or treatment failure has not been elucidated [Reference Yu12, Reference Pallares22]. In fact, most studies [Reference Moroney11, Reference Yu12, Reference Song14] failed to find a significant correlation between penicillin resistance and increased mortality. In addition, Weinstein et al. [Reference Weinstein, Klugman and Jones23] detected no relationship between mortality and penicillin MIC ⩾2 μg/ml in patients treated with penicillin alone. These results, along with a review of pharmacokinetic and pharmacodynamic data [Reference Craig24], led to the decision in 2008 to revise the penicillin breakpoints. According to the new guidelines [16], the breakpoints for susceptible, intermediate, and resistant in non-meningitis cases are ⩽2, 4, and ⩾8 μg/ml, respectively. For meningitis cases there is no intermediate category; isolates are categorized as susceptible (⩽0·06 μg/ml) or resistant (⩾0·12 μg/ml). In this study, we found that even with the revised penicillin susceptibility breakpoints, clinical outcomes in patients infected with Pen-NS strains were no different from those in patients infected with Pen-S strains. We believe that this result provides encouragement to clinicians to prescribe penicillin with more confidence for non-meningeal pneumococcal infections.
The high prevalence and levels of resistance to erythromycin in our study are consistent with what has been reported from other Asian countries [Reference Song2, Reference Song13, Reference Choi and Lee25, Reference Hsueh26]. It has also been noted that the frequency of resistant pneumococcal isolates containing both erm(B) and mef(A) is higher in South Korea than in other regions [Reference Waites27], which might be partly due to the clonal spread of a few resistant clones [Reference Ko and Song28]. Over the past decades, there have been several reports of macrolide treatment failures in patients with pneumonia or bacteraemia infected with erythromycin-resistant S. pneumoniae [Reference Kelley7–Reference Van Kerkhoven9]. Given the fact that treatment failure is, to some extent, dependent on levels of resistance, it is imperative to examine the clinical significance of macrolide resistance in the light of the dominant mechanisms of resistance in the study population.
In the present study, we found that macrolide resistance did not result in increased mortality. Similarly, in two studies [Reference Song14, Reference Aspa29] in which researchers examined the impact of macrolide resistance, mortality in patients with erythromycin-resistant pneumococcal pneumonia was not significantly different from that in patients with erythromycin-susceptible pneumococcal pneumonia. However, both studies had limitations in that they included isolates from respiratory specimens, which might have obscured a real impact of resistant strains on clinical outcomes.
The 30-day mortality of 26·7% is comparable to that reported in other studies of pneumococcal bacteraemia (10–30%) [Reference Yu12, Reference Yu14, Reference Yu30–Reference Song34]. Our data revealed that the independent risk factors for 30-day mortality in patients with pneumococcal bacteraemia were a high APACHE II score and presence of a solid organ tumour. Similarly, previous studies that emphasized the importance of host factors in predicting outcome have demonstrated that severity of illness, advanced age, and comorbidities such as malignancy, are significantly associated with mortality [Reference Yu12, Reference Kim30, Reference Turett32, Reference Feikin35]. Our results support the idea that factors other than resistance, such as severity of illness at presentation and underlying disease, have a stronger influence on clinical outcomes.
It is noteworthy that patients infected with EM-NS strains had longer times to defervescence. Some [Reference Metlay4, Reference Metlay36] have argued that of the various outcome measures, mortality may not be sensitive to differences in drug efficacy and may be confounded by other factors, although it is believed to be the most reliable and clinically relevant measure. Other outcome measures such as bacterial eradication, rate of complication, and time to stability, might be more relevant endpoints for exploring the relationship of the impact of virulence on medical outcomes.
It remains unclear what sort of mechanisms may have contributed to the delayed time to defervescence in the EM-NS group. As we did not adjust further for other variables associated with delayed resolution of fever, investigation of other possible confounders such as host factors and pathogen-specific factors that affect the intrinsic virulence of the organisms (e.g. capsular subtype) is needed to determine whether antibiotic resistance affects the time required for defervescence.
To the best of our knowledge, this is the first study using the new CLSI guidelines to investigate the impact of penicillin and erythromycin resistance on clinical outcomes in adult patients with pneumococcal bacteraemia. However, some limitations of the study should be noted. First, it focused on mortality as the primary endpoint, and, as noted earlier, mortality may be a relatively insensitive measure for establishing the impact of drug resistance on medical outcomes. However, in contrast with the other studies, we did our best to examine other potential outcomes such as time to fever resolution, infection-related complications (shock, admission to ICU, pleural effusion), and length of hospital stay. Second, it was an observational study, so the types of antibiotics administered could not be controlled. Finally, the results may not be applicable to outpatients or hospitalized patients without bacteraemia.
In summary, we found that having a severe underlying disease was an independent risk factor for 30-day mortality. On the other hand, antimicrobial resistance, even high-level resistance, did not affect clinical outcomes in adult patients with pneumococcal bacteraemia, although EM-NS was associated with delayed resolution of fever in patients with bacteraemic pneumococcal pneumonia. Our findings support the notion that clinical outcomes are more closely related to clinical condition at presentation than to level of antimicrobial resistance.
ACKNOWLEDGEMENTS
The work was supported by grant no. 04-2008-020-0 from the research fund of Seoul National University Hospital.
DECLARATION OF INTEREST
None.







