Text short report
Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBLs) are an important reason for therapy failure with β-lactam antibiotics [Reference Pitout and Laupland1]. Infections due to ESBL-producing Enterobacteriaceae (ESBL-E) are often preceded by asymptomatic carriage [Reference Freeman2]. Besides human-to-human transmission, potential routes of transmission of ESBL-E to humans are the food chain, direct contact with animals or indirectly via the environment [Reference Huijbers3]. The prevalence of the faecal carriage of ESBL-E in the general population in the Netherlands is approximately 5% [Reference Huijbers4–Reference van den Bunt6].
With enteric zoonotic diseases (including Salmonellosis, Campylobacteriosis and infections with Shiga toxin-producing Escherichia coli), seasonal variation is ubiquitous in temperate regions, with increased human disease incidence during warmer months [Reference Lal7]. In general, this increased incidence is following the increased pathogen prevalence and shedding from animal reservoirs during warmer months [Reference Lal7]. Indeed, during warmer months, often an increase in the environmental presence of E. coli is observed [Reference Liu, Hofstra and Franz8]. On the other hand, higher temperature is associated with reduced E. coli survival in water and soil, which is probably related to the increased competition for nutrients following the increased activity of environmental bacteria at higher temperatures [Reference Franz9].
Seasonality in healthcare-associated infections, mainly bloodstream infections (BSIs), caused by Gram-negative bacteria including E. coli and Klebsiella pneumoniae has been shown in several studies with higher BSI incidence rates with temperature increase, although for K. pneumonia this association was not always observed [Reference Richet10]. More recent studies showed that mainly community-acquired E. coli BSIs, in contrast to hospital-acquired BSIs, showed higher rates during warmer months [Reference Gradel11, Reference Deeny12]. However, ESBL-E was not assessed in these studies. A recent ecological study on antibiotic resistance in E. coli, K. pneumoniae and Staphylococcus aureus using antibiotic resistance pattern data from hospitals, laboratories and surveillance units in the USA showed that the prevalence of antibiotic resistance increases with local temperature (increase in average minimum temperature) [Reference MacFadden13].
So far, seasonal variation in asymptomatic ESBL-E carriage in the general population has not been investigated. Two studies investigated the seasonal trends of ESBL-carriage in patients. One study by Mesa et al. on the faecal carriage of ESBL-E in a limited number of patients attending an emergency room of two hospitals in Barcelona and persons involved in food-borne outbreaks showed that there was a temporal distribution in ESBL-E carriage with a decrease in the prevalence in May and an increase in July [Reference Mesa14]. However, data were solely descriptive without adjustment for confounders such as previous travel or antibiotic use, and sampling was not performed year-round. A second study by Kaier et al. assessed the incidence of carriage (including both colonised persons and persons with infections) of ESBL-producing E. coli and K. pneumoniae (ESBL-E/K) in hospitalised patients in two German university hospitals by using routine laboratory surveillance data. They found an increasing trend in the incidence of ESBL-E/K carriage during summer compared to winter [Reference Kaier15].
It is important to know whether seasonality (or monthly variations) is present in the asymptomatic carriage of ESBL-E in order to design future prevalence studies more appropriately by decreasing the chance of finding factors associated with ESBL-E carriage which is due to a difference in exposure (i.e. moment of sampling). The aim of the present study is to assess whether there is a seasonal difference in the carriage of ESBL-E/K in the general Dutch population. The outcomes of this pooled analysis may also help to better understand the variations in ESBL-E/K carriage rates.
From 2014 to 2017, the faecal carriage of ESBL-producing E. coli and K. pneumoniae was determined in three different large cross-sectional studies in the Netherlands in the selections of predominantly healthy individuals in the population at large: the Livestock Farming and Neighbouring Residents' Health Study (VGO) [Reference Wielders5], the ESBL-population study (ESBLAT) [Reference van den Bunt6] and a study on ESBL-producing bacteria among vegetarians and non-vegetarians (Vega Study) [Reference Meijs16]. The VGO study was performed from February 2012 to May 2014 among the general population aged 18–70 years old living in a livestock-dense area in the south of the Netherlands, and after consent to participate in further studies, ESBL-E/K faecal carriage was assessed between February 2014 and May 2015 [Reference Wielders5]. In the ESBLAT study, a random sample of 2000 persons was invited every month from the Dutch Personal Records Database to submit faecal samples between October 2014 and February 2017. In the Vega Study, vegetarians and persons eating meat were actively contacted to submit faecal samples in order to study ESBL-E/K carriage between November 2015 and March 2017. More characteristics of the three studies are described in Table 1 and details of the respective studies and laboratory methods can be found in the cited references [Reference Wielders5, Reference van den Bunt6, Reference Meijs16]. All individual level data of the three studies were combined into a new dataset in order to perform a pooled analysis. Pooling was carried out since the participants in all three studies were sampled from the general population, were aged ≥18 years and similar laboratory techniques to identify ESBL-E/K carriers were performed. Participants were only included in the analysis when (i) aged ≥18 years, (ii) data on antibiotic use in the last 6 months were available and (iii) data on travelling in the last 6–12 months were available. Two participants of the ESBLAT study carrying ESBL-producing Enterobacter cloacae complex were excluded from our analyses. Data on antibiotic use and travel were collected via self-reported questionnaires in all three studies, except for antibiotic use in the VGO study. In the VGO study, antibiotic use was derived from general practitioner (GP) electronic medical records and data were only included when the GP registered prescriptions for ≥46 weeks during the calendar year and if the patient was registered at the particular GP for at least three-quarters of the year [Reference Wielders5]. Data on travelling in the last 6 months were available for participants from the Vega Study and for the last 12 months for the VGO and ESBLAT studies (Table 1). Of note, vegans and vegetarians participating in the Vega Study were excluded from the pooled analysis, since including them would result in a non-representative sample of the adult general Dutch population, as around 95% of the population eats meat. We therefore only included participants from the Vega Study who reported to eat meat at least once per week. However, our analysis still included vegetarians that participated in the VGO and ESBLAT studies.
Table 1. Characteristics of the cross-sectional studies performed in the Netherlands (2014–2017) and included in the pooled analysis

a Exclusion because of missing data on antibiotic use from general practitioners (n = 392), missing data on travel (n = 20) or both antibiotic use and travel data missing (n = 8).
b Exclusion because of age <18 years (n = 574), unknown date of sampling (n = 2), unknown age (n = 1), ESBL-producing Enterobacter cloacae complex (n = 2), missing data on antibiotic use (n = 20), missing data on travel (n = 19) or both antibiotic use and travel data missing (n = 2).
c Exclusion because of not eating meat (n = 1177), eating meat less than once per week (n = 42) or missing data on travel (n = 1).
Results from the three studies were pooled to study the presence of seasonal trends in prevalence throughout the year (by month of sampling). The trend in monthly prevalence was tested using the Mantel–Haenszel χ 2 test. A P-value <0.05 was considered statistically significant. Univariate and multiple logistic regression analysis was performed and pooled odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated. All variables available were included into the multivariate analysis since these were either general characteristics (age, sex), known risk factors for ESBL-E/K carriage (antibiotic use during the last 6 months and travel during the last 6–12 months) or our variable of interest (month of sampling). To correct for multiple testing, the Benjamini–Hochberg procedure with a 10% false discovery rate was used. Furthermore, a supplementary analysis was executed to assess if the pooled logistic regression analysis was justified compared to a multilevel logistic regression model that corrects for between-study variation (by using study as a random effect). All analyses were performed using SAS (version 9.4).
Furthermore, we also checked data from the Dutch national AMR surveillance system, based on routine data from medical microbiological laboratories [Reference Altorf-van der Kuil17], to investigate if there was a seasonal trend in the monthly prevalence of ESBL-E/K BSIs (i.e. the proportion of BSIs caused by ESBL-E/K among all BSIs caused by E. coli/K. pneumoniae) from isolates taken between 2014 and 2016. ESBL production was estimated by ESBL confirmatory tests, or, when no data on confirmatory tests were available, by resistance against cefotaxime/ceftriaxone and/or ceftazidime.
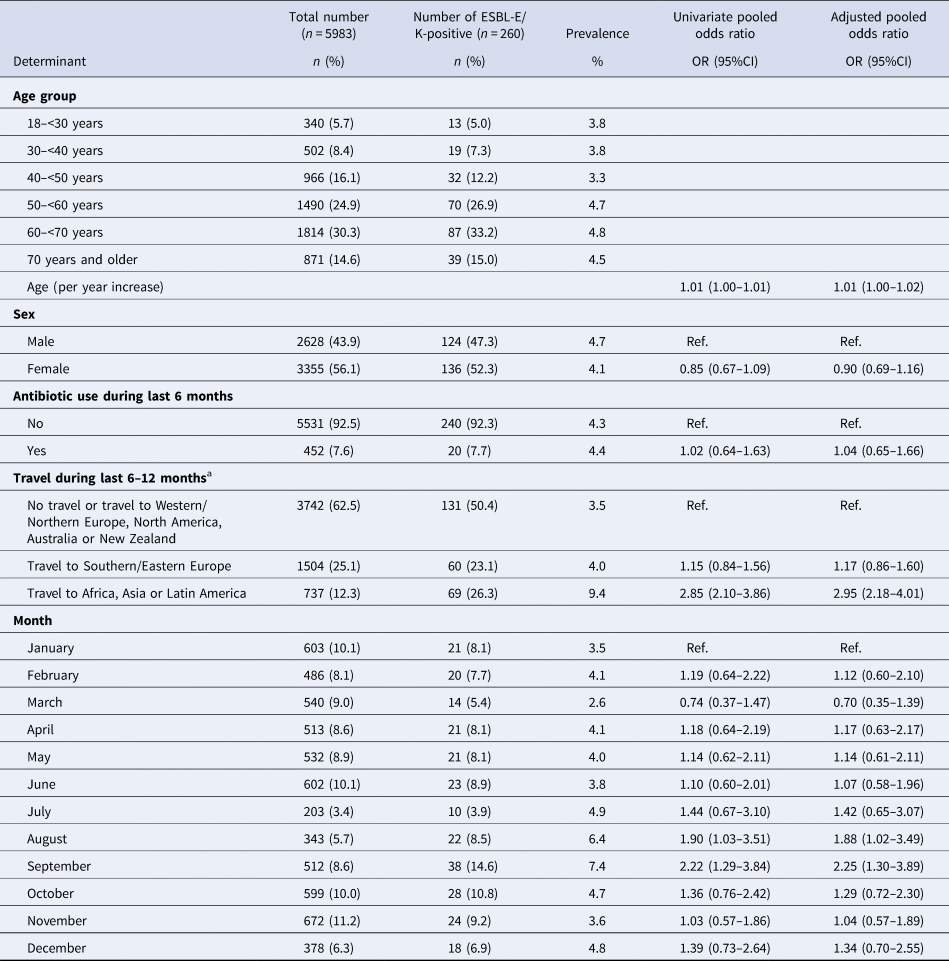
A total of 8636 faecal samples of an equal amount of persons had been analysed in the three studies, and 5983 (69.3%) fulfilled the criteria to be included in this pooled analysis with a median age of 58 years (interquartile range 47–66) (Table 1). Of those, 260 tested positive for ESBL-E/K (4.3%; 95% CI 3.9–4.9%; range 2.6–7.4% per month). The month of sampling showed a statistically significant trend indicating that the observed prevalence differed between the months of the year (Mantel–Haenszel χ 2P-value of 0.036). In univariate analysis, monthly differences in the prevalence of ESBL-E/K carriage were observed in our data, with the highest prevalence in August and September compared to January (Table 2). In addition, travel to Africa, Asia or Latin America was statistically significant and associated with an increase in the ESBL-E/K prevalence (OR 2.85, 95% CI 2.10–3.86). The Benjamini–Hochberg procedure showed that travel to Africa, Asia or Latin America and September remained statistically significant. In multiple logistic regression analysis, the seasonal differences in the prevalence of ESBL-E/K carriage from the univariate analysis remained almost the same when results were adjusted for age, sex, previous antibiotic use and travel: compared to January, the odds of being an ESBL-E/K carrier was 1.88 in August (95% CI 1.02–3.49) and 2.25 in September (95% CI 1.30–3.90). When analyses were repeated without adjusting for antibiotic use during the previous 6 months (to increase the number of participants and ESBL-E/K carriers (n = 6395 participants of whom 291 (4.8%) were the carrier of ESBL-E/K)), results were very similar with a little increase in the ORs for August and September (data not shown). The random-effect estimates for the study were not statistically significant in the supplementary analysis, indicating that there was no significant variation between the studies. The ORs from the multilevel model were very similar to the ORs from the pooled logistic regression analysis.
Table 2. Descriptive characteristics, univariate and multiple logistic regression analysis of three pooled cross-sectional studies on the ESBL-E/K prevalence in the general Dutch population
a Depending on the study.
Finally, no clear seasonal trend could be observed in the monthly ESBL/E-K BSI prevalence data from the national antimicrobial resistance surveillance system: all Mantel–Haenszel χ 2P-values were non-significant, both overall and when a distinction was made between hospital-acquired (blood sample taken >2 days after admission) and community-acquired BSIs (data not shown).
In this pooled analysis with the data of three consecutive years (February 2014–April 2017) from 5983 individuals, ESBL-E/K carriage rates in the general population varied over the months of the year. Adjustment for age, sex and known risk factors for ESBL-E/K carriage (travel and antibiotic use) negligibly influenced the results from the univariate analysis and other factors seem to play a more important role to the exposure. We hypothesise that the higher ESBL-E/K carriage rate in August and September is caused by an increased chance of exposure to and/or acquisition of ESBL-E/K, potentially in combination with other factors including human behaviour/social and environmental factors.
As described above, increased incidence of enteric zoonotic diseases during warmer months including Salmonellosis, Campylobacteriosis and infections with Shiga toxin-producing E. coli has been reported in temperate regions [Reference Lal7]. This seasonality during warmer months also seems to be applicable to infections caused by E. coli and ESBL-E/K, while for non-ESBL-producing K. pneumoniae, conflicting results were found between different studies with regard to seasonal variations [Reference Richet10, Reference Kaier15]. Recent new insights into the distinction in seasonality between hospital-acquired and community-acquired BSIs, with only higher rates during warmer months for community-acquired BSIs and not for hospital-acquired infections [Reference Gradel11, Reference Deeny12], suggest the existence of a certain mechanism for infection outside the hospital. Moreover, seasonality seems to be present in infections and carriage caused by ESBL-E as shown previously [Reference Mesa14, Reference Kaier15]. Interestingly, the study by Gradel et al. on community-acquired bacteraemia caused by E. coli in Denmark observed peak dates for different subgroups in August and September [Reference Gradel11], which are exactly the same months of increased ESBL-E/K carriage in the community in the present pooled analysis.
Several explanations for increased rates of infections and ESBL carriage of E. coli and K. pneumoniae during summer in humans have been suggested. However, the causal mechanisms driving this are generally unknown and different processes seem to counteract each other, like increased animal shedding vs. decreased survival outside the host with increasing temperatures. In addition, it is very likely that it is an interplay between environmental and human behavioural/social factors. The abundance and diversity of genes encoding ESBLs differ significantly between open community and livestock and their products, indicating that animal production and foodborne transmission have a lesser contribution to ESBL carriage in humans than previously assumed in the Netherlands [Reference Dorado-Garcia18], and human-to-human contact is the main driver for ESBL-E transmission [Reference Dorado-Garcia18–Reference Mughini-Gras20]. Therefore, increased human–human exposure seems to be the most likely cause for the higher ESBL-E/K carriage rate in certain months. Increased transmission at population-level may consequently enable population-level selection of resistant strains [Reference MacFadden13]. Nevertheless, during warmer months, human exposure to contaminated food (i.e. undercooked meat) may be increased by different eating habits and diminished food hygiene (like during BBQs, picnics and eating more raw vegetables). Besides, increased exposure via environmental sources (like water and soil) and via animals could also play a role [Reference MacFadden13], potentially in combination with seasonal human behaviour such as performing more outdoor activities (i.e. beach and swimming in recreational waters), more frequent contact with family and friends, and increased sexual activity during summer [Reference Freeman, Anderson and Sexton21]. However, the attribution of ESBL-carriage to environmental sources and contact with animals seems to be limited [Reference Mughini-Gras20], which might explain why no effect of study (i.e. one of the studies was in a livestock-dense area) on the monthly variations was found in our analyses. In addition, seasonal changes in susceptibility to colonisation or infection are also possible. For example, there is an indication that photoperiod (day length)-driven physiologic changes are occurring in mammalian species, including some in humans. This possibly has an influence on the susceptibility of the host population for certain pathogens [Reference Dowell22]. Furthermore, higher temperatures may facilitate the uptake of free genetic material or horizontal gene transfer, which is the case for plasmid-mediated ESBLs [Reference MacFadden13]. This increased gene transfer (in the host or the environment) might then be likely to facilitate population transmission [Reference MacFadden13]. Moreover, MacFadden et al. found that fluoroquinolones and β-lactams in general showed the strongest associations between temperature and resistance, which supports the mechanism-specific impacts of temperature on resistance [Reference MacFadden13]. Finally, Davenport et al. showed in a population with a minimal environmental and genetic variation that despite overall gut microbiome stability within individuals over time, there are consistent and significant population-wide shifts in microbiome composition across seasons. In general, a lower gut microbiome diversity was observed during summer than during winter [Reference Davenport23]. This seasonal shift in microbiome composition might play a role in the chance of individuals to become colonised with ESBL (i.e. their susceptibility); however, at the moment this is still unclear.
The current analysis was performed by pooling data from three different cross-sectional studies in the Netherlands using univariate and multiple logistic regression analysis. All participants were from the general population, aged ≥18 years and similar laboratory techniques to identify ESBL-E/K carriers were performed. The supplementary analysis with random effects for the study did not alter our results. It was therefore decided to keep the analysis as simple as possible.
A limitation of the study is that we only had data available of reported travel during the last 6–12 months (depending on the study), and we did not know the exact timing of the reported travel relative to the faecal sampling. Therefore, we might not have been able to adjust our analyses completely for travel. A second limitation is that, although data were available for three consecutive years, the number of samples for some months in this period was too low to perform a longitudinal analysis check whether the effect observed in August and September occurred yearly.
Finally, the analysis of the national surveillance data showed no clear seasonal trend in the monthly proportion of BSIs caused by ESBL-E/K out of all E. coli and K. pneumoniae BSIs. A limitation of this analysis was, however, that the distinction between hospital-acquired and community-acquired infection based on the surveillance data might have been biased as the date of hospitalisation was missing in a substantial part of the infections. Furthermore, we could not include incidence data on ESBL-E/K BSIs because no monthly denominator is available in the surveillance system on routine diagnostics.
In conclusion, differences in monthly ESBL-E/K carriage rates in the general community were found in the Netherlands, which may indicate that the factors during warmer seasons could influence the probability of an individual to become colonised. However, no seasonal trend in the proportion of ESBL-E/K BSIs was seen in the national surveillance data, indicating that the increased ESBL-E/K carriage rate did not result in an increase in community-onset invasive infections. In addition to adjusting for known risk factors, we advise to consider seasonal fluctuations in carriage rates when designing future ESBL prevalence studies in the general community and among risk groups (such as persons in nursing homes, hospitals) in temporal regions, as these might partly adjust for human behavioural/social and environmental factors that affect ESBL-E/K carriage in the community. We therefore recommend to sample participants within the same time window (i.e. during the same season but preferably (the same fraction) year-round) and not consecutively.
Acknowledgements
We would like to thank all participants and persons that were involved in the ESBLAT, VGO and Vega Study for analysing all samples.
Financial support
The Livestock Farming and Neighbouring Residents' Health (VGO) study was funded by the Ministry of Health, Welfare and Sports and the Ministry of Economic Affairs of the Netherlands, and supported by a grant from the Lung Foundation Netherlands (Grant number: 3.2.11.022). ESBL-population study (ESBLAT) was supported by the Dutch public private partnership 1Health4Food (TKI AF-12067, ESBL Food Attributive Risks) and by the Dutch Ministry of Health, Welfare, and Sport. The study on ESBL-producing bacteria among vegetarians and non-vegetarians (Vega Study) was funded by the Ministry of Health, Welfare and Sports of the Netherlands.
Conflict of interest
The authors do not have any conflicts of interest.




