Under the umbrella category of autism spectrum disorder (ASD) are included neurodevelopmental conditions with a high genetic and phenotypic heterogeneity, but shared by a symptom dyad: (1) deficits in social communication and interactions; and (2) repetitive patterns of behaviour, interests and activities (Diagnostic and Statistical Manual of Mental Disorders, DSM-5; American Psychiatric Association, 2013). The modifications of the diagnostic criteria included in the current version of DSM have enhanced the role of the second domain: in fact, the restricted and repetitive behaviours (RRB), once considered non-ASD specific and subordinate to the socio-communication deficit, are now required for an ASD diagnosis.
Recent ASD research attempted to investigate whether the social communication and RRB dimensions are correlated with distinct endophenotypes and genotypes. In fact, a number of family studies in ASD have shown that genes controlling RRB are independent of genes controlling social or communication impairments (Mandy & Skuse, Reference Mandy and Skuse2008). Similarly, among the neuroanatomical correlates of ASD symptoms (Bellani et al. Reference Bellani, Calderoni, Muratori and Brambilla2013a, Reference Bellani, Calderoni, Muratori and Brambillab), RRB have been frequently associated with structural alterations of the basal ganglia (BG), the deep grey matter structures that include the caudate nucleus, the putamen and the globus pallidus. Several structural magnetic resonance imaging (sMRI) studies have investigated the BG volumetry in patients with ASD and its correlation with the RRB severity. The association between BG structure and RRB symptom expression has been frequently, but not always (Hardan et al. Reference Hardan, Kilpatrick, Keshavan and Minshew2003), found (see Table 1) and calls for further inquiry. First, (a) since the RRB domain encompasses a broad range of symptoms, ranging from repetitive motor behaviours (‘lower order’ RRB) to restricted behaviours and resistance to change (‘higher order’ RRB), future studies should investigate if different RRB phenotypes are related to different neuroanatomical underpinnings. Second, BG alterations are present in other neuropsychiatric conditions (e.g. attention deficit-hyperactivity disorder, social anxiety or obsessive-compulsive disorders): studies that compare ASD individuals with those affected by the above-mentioned disorders could contribute to define the specific characteristics of BG involvement in ASD; and finally, the effect of potentially significant factors other than RRB (age, gender, cognitive and adaptive functioning, ASD symptom severity, comorbid psychopathology, psychotropic medications and pattern of ASD onset) on BG volumes of ASD patients has not been clearly elucidated yet: longitudinal research designs in larger sample of carefully assessed subjects could contribute to this aim.
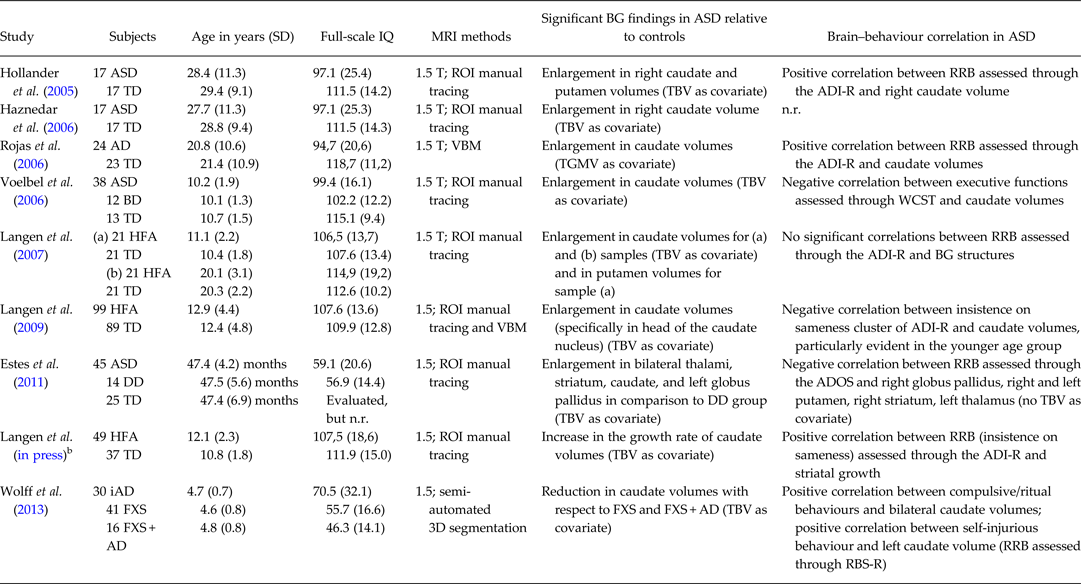
Table 1. Summary of studies published between 2005–2013 investigating basal ganglia volumetry in patients with autism spectrum disorders (ASD) compared with control subjectsa

AD, autistic disorder; ADI-R, Autism Diagnostic Interview-Revised; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorders; BD, bipolar disorder; BG, basal ganglia; DD, developmental delay; FXS: fragile X syndrome; FXS + AD, fragile X syndrome with autistic disorder; HFA, high-functioning autism; iAD. idiopathic autistic disorder; n.r., not reported; RBS-R, Repetitive Behaviour Scales-Revised; RRB, restricted and repetitive behaviours; TBV, total brain volume; TD, typically developing control subjects; TGMV, total grey matter volume; VBM, voxel-based morphometry; WCST, Wisconsin Card Sorting Test.
aDue to the editorials guideline of limited number of references, only the most recent MRI studies on basal ganglia volumetry in ASD were considered, starting from year 2005.
bLongitudinal study.
In addition to the fact that RRB are socially inappropriate and stigmatising, they also interfere with acquisition of adaptive and social skills. Moreover, RRB affect considerably day-to-day functioning and can jeopardise optimal academic and vocational placement. Recent research has focused on methods for reducing RRB, by triggering a positive cascade of effects on other behaviours. Therefore, elucidating the neurobiological underpinnings of the RRB will increase our understanding of the system involved and would eventually facilitate the development of effective intervention strategies. First, since BG volumetry is related to RRB severity, future longitudinal studies should explore the potential use of BG sMRI analysis as an adjunct method for monitoring the response to intervention aimed at reducing RRB. Second, additional research is needed to identify the brain circuitry associated with different functions of RRB in individuals with ASD (e.g. self-stimulation, modulation of anxiety) in order to define tailored treatment strategies that target those pathways. Third, the integration of results from different MRI techniques (i.e. Diffusion Tensor Imaging, proton spectroscopy, resting state fMRI and functional MRI) or newer imaging analysis methods (e.g. Support Vector Machines) applied to the same subjects could enhance our knowledge on symptom-specific neurobiological underpinnings, paving the way for treatment development.
In conclusion, research into the contribution of BG abnormalities to RRB pathogenesis and pathophysiology is an emerging and challenging new area that compliments and extends the search for ASD neuroanatomical correlates, with the final aim of translating laboratory findings into clinical practice.
Financial Support
S. C. was partly supported by the Italian Ministry of Health and by Tuscany Region with the grant ‘GR-2010-2317873’. F. M. and S. C. were partly supported by the European Union (The MICHELANGELO Project). The other authors received no specific grant from any funding agency, commercial or not-for-profit sectors for this publication.
Conflict of Interest
None.
Ethical Standard
The authors declare that no human or animal experimentation was conducted for this work.