Introduction
Major depressive disorders have an increasing impact on the global burden of disease and are highly prevalent in the global population (4.4%) (G. B. D. Disease Injury Incidence Prevalence Collaborators, 2016; World Health Organization, 2017). Findings from the Global Burden of Diseases, Injuries and Risk Factors Study in 2015 (GBD 2015) show that major depressive disorders ranked third among the leading causes of disability in the world (G. B. D. Disease Injury Incidence Prevalence Collaborators, 2016). Despite the burden of these disorders, their correlation with other medical conditions like chronic diseases tends to be underestimated (Prince et al., Reference Prince, Patel, Saxena, Maj, Maselko, Phillips and Rahman2007). These findings highlight that depressive disorders are a current issue for public health and will be a future challenge for policymakers.
Although criticised for only considering costs and not effects, information on health care costs as provided by cost-of-illness (COI) studies can be useful to emphasise the economic relevance of a disease (Koopmanschap, Reference Koopmanschap1998; Larg and Moss, Reference Larg and Moss2011). These studies can be classified according to two methodological approaches: In bottom-up studies, costs of patient samples are assessed on basis of individual resource-consumption, whereas in top-down studies, aggregate costs at population-level are combined with relative risk and prevalence rates of a disease. Disease-specific costs can be extracted from bottom-up studies by matching a non-diseased comparison group and calculating excess costs (the difference between the costs of diseased and non-diseased patients) (Akobundu et al., Reference Akobundu, Ju, Blatt and Mullins2006; Larg and Moss, Reference Larg and Moss2011).
Previous systematic reviews of COI-studies of depression addressed specific subtypes or age groups or the costs of depression as comorbidity of somatic diseases (Lehnert et al., Reference Lehnert, Konnopka, Riedel-Heller and König2011; Luppa et al., Reference Luppa, Sikorski, Motzek, Konnopka, König and Riedel-Heller2012; Molosankwe et al., Reference Molosankwe, Patel, José Gagliardino, Knapp and McDaid2012; Mrazek et al., Reference Mrazek, Hornberger, Altar and Degtiar2014; Sambamoorthi et al., Reference Sambamoorthi, Shah and Zhao2017). The last global systematic review was conducted in 2007 (Luppa et al., Reference Luppa, Heinrich, Angermeyer, König and Riedel-Heller2007). In general, a large number of systematic reviews with cost data are available in the literature, but very few conducted meta-analyses (van der Hilst et al., Reference van der Hilst, Ijtsma, Slooff and Tenvergert2009; Haschke et al., Reference Haschke, Hutter and Baumeister2012; Zhang et al., Reference Zhang, Li, Hong, Xu, Liu, Huang and Liu2018). Haschke and colleagues included depression in a meta-analysis of COI-studies of coronary artery disease and coexistent mental disorders, but none was solely focusing on depression. Reasons for comparatively little literature on meta-analyses with cost data could be that combining results across studies is difficult and requires a specific format, namely costs reported for a diseased and non-diseased group.
This study is a systematic review and meta-analysis of bottom-up COI-studies of depression with comparator group, with the objectives to (1) update and provide a global overview of the current state of the literature (2) assess the impact of depression on costs by calculating effect sizes of included studies (3) conduct a meta-analysis and display pooled results of all studies as forest plots (4) draw generalizable conclusions about the relevance of depression.
Methods
We used the 27-item checklist of the PRISMA Statement as guideline for this systematic review and meta-analysis (Liberati et al., Reference Liberati, Altman, Tetzlaff, Mulrow, Gotzsche, Ioannidis, Clarke, Devereaux, Kleijnen and Moher2009). Studies were considered for inclusion if they met the following eligibility criteria: full-text peer-reviewed articles in English or German reporting costs for depression and a comparison group were included. We included bottom-up studies, with no limitation on publication date and study design. Reviews, commentaries, editorials, short reports, and duplicates were excluded. Participants with a diagnosis of depression (e.g. major depressive disorder, mild depression, depressive symptoms) were included. If two patient samples (e.g. in the depressed patient group) were reported, they were pooled to a single patient group (Higgins and Deeks, Reference Higgins and Deeks2011). Exclusion criteria were participants with bipolar disorders, adjustment disorders or other mental disorders like anxiety disorders. Studies with patient subgroups, but missing information needed for pooling (standard deviation (s.d.), sample sizes) were also excluded. No limitations on age, region or diagnostic instruments were imposed. For the purpose of this study, a depressed group is compared to a non-depressed group. Studies comparing excess costs of depressed and non-depressed among (1) adolescent, adult and elderly participants or (2) participants with a specific primary diagnosis of a somatic disease were included. The outcome of interest was limited to studies reporting mean costs for both groups in monetary units per participant. Outcomes only reported as median, log mean or mean difference, predicted costs and results from two-part models were excluded. If both were reported, unadjusted means were preferred over adjusted means. The reason was that processed data contains the risk of an additional source of variability between studies.
We conducted a systematic literature search in PubMed, PsycINFO, NHS Economic Evaluation Database and EconLit following the search term of the most recent systematic review on COI-studies of depression (‘cost*’ OR ‘economic burden’ OR ‘cost-of-illness’ OR ‘burden-of-illness’) AND (‘depression’ OR ‘depressive disorder’) (Luppa et al., Reference Luppa, Heinrich, Angermeyer, König and Riedel-Heller2007). Additionally, reviews and references in identified articles were screened for more relevant literature. The initial search was conducted by HK and completed on 30/01/2018. Literature was then searched for updates until 27/04/2018. Search results were screened for eligibility by title and abstract and then retrieved for full-text examination. Eligibility assessment was performed by HK and AK and in the case of disagreement the reasons were discussed until agreement on eligibility was achieved. Data were identified and extracted in a piloted Excel sheet by HK and double checked by a second reviewer. Authors were contacted if data were missing or unclear for selection of articles.
Data on (1) study characteristics (study year, country, study perspective and data source) (2) participants (sample sizes, age range, diagnostic instruments, diagnostic criteria and included disorders) (3) characteristics of the depressed and non-depressed group and (4) outcome (year of pricing, currency and time interval for costs) were extracted from included articles. We created cost categories for direct excess costs (inpatient, emergency, outpatient treatment, medication and a category including all other direct costs) and indirect excess costs (reduced/lost productivity). If more than one outcome was reported per cost category, we summed mean values and imputed standard errors (s.e.) in the meta-analysis. The methodological quality of included studies was assessed independently by two reviewers. Since there was no existing standardised checklist for COI, we used the checklist reported by Stuhldreher et al. (Reference Stuhldreher, Konnopka, Wild, Herzog, Zipfel, Löwe and König2012), see online Supplementary material S1.
Costs across studies were adjusted to a 12 month time interval, inflated to the year 2017 using consumer price indices and converted to US dollars using Purchasing Power Parities (US$ PPP). For missing data on the year of pricing, we made assumptions based on the recruitment period or information provided in the text and other sources. We formed four patient subgroups for comparison (depressed v. non-depressed in adolescents, adults, old age and depression as comorbidity).
We used Ratio of Means (RoM) as effect measure in the meta-analysis, which is calculated as the mean of the depressed group divided by the mean of the non-depressed group (Friedrich et al., Reference Friedrich, Adhikari and Beyene2008; Reference Friedrich, Adhikari and Beyene2011). Results are interpreted as the percentage change in the depressed group compared to the non-depressed group (e.g. RoM = 1.15 implies that the mean costs of the depressed group are 15% higher than the comparison group) (Fu et al., Reference Fu, Vandermeer, Shamliyan, O'Neil, Yazdi, Fox and Morton2014). RoM and corresponding s.e. were calculated in Excel and log-transformed for pooling. Using Review Manager 5.3, results of studies were combined with the generic inverse variance method (DerSimonian and Laird) using random-effects models (DerSimonian and Laird, Reference DerSimonian and Laird1986). When s.d. was missed, we imputed data using direct substitution of the highest s.e. in the patient subgroup (Fu et al., Reference Fu, Vandermeer, Shamliyan, O'Neil, Yazdi, Fox and Morton2014). Pooled results are back-transformed so that RoM and 95% confidence intervals (95% CI) are presented on a non-logarithmic scale (Friedrich et al., Reference Friedrich, Adhikari and Beyene2008, Reference Friedrich, Adhikari and Beyene2011). Heterogeneity was assessed with I 2 statistic (with I 2 = 25%, I 2 = 50% and I 2 = 75% indicating low, moderate, and high heterogeneity) (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003). Meta-analyses were performed for direct and indirect total excess costs as well as for all cost categories separately. All eligible studies were included in the systematic review and meta-analysis of total excess costs. In the direct cost categories, meta-analyses were conducted if more than one study was comprised in the patient subgroups. Results of meta-analysis are shown as forest plots. Robustness of results was tested by removing studies with extreme values from the analysis.
Results
We identified 12 760 articles and 37 additional articles through references to studies. After exclusion of duplicates, supplemental material and non-English or German literature, we screened 11 405 articles by title and abstract, of which 11 012 were excluded. Of 393 full-text articles assessed for eligibility, 345 were excluded, because they did not meet the inclusion criteria (see Fig. 1). In total, 48 studies were included in the systematic review and meta-analysis.
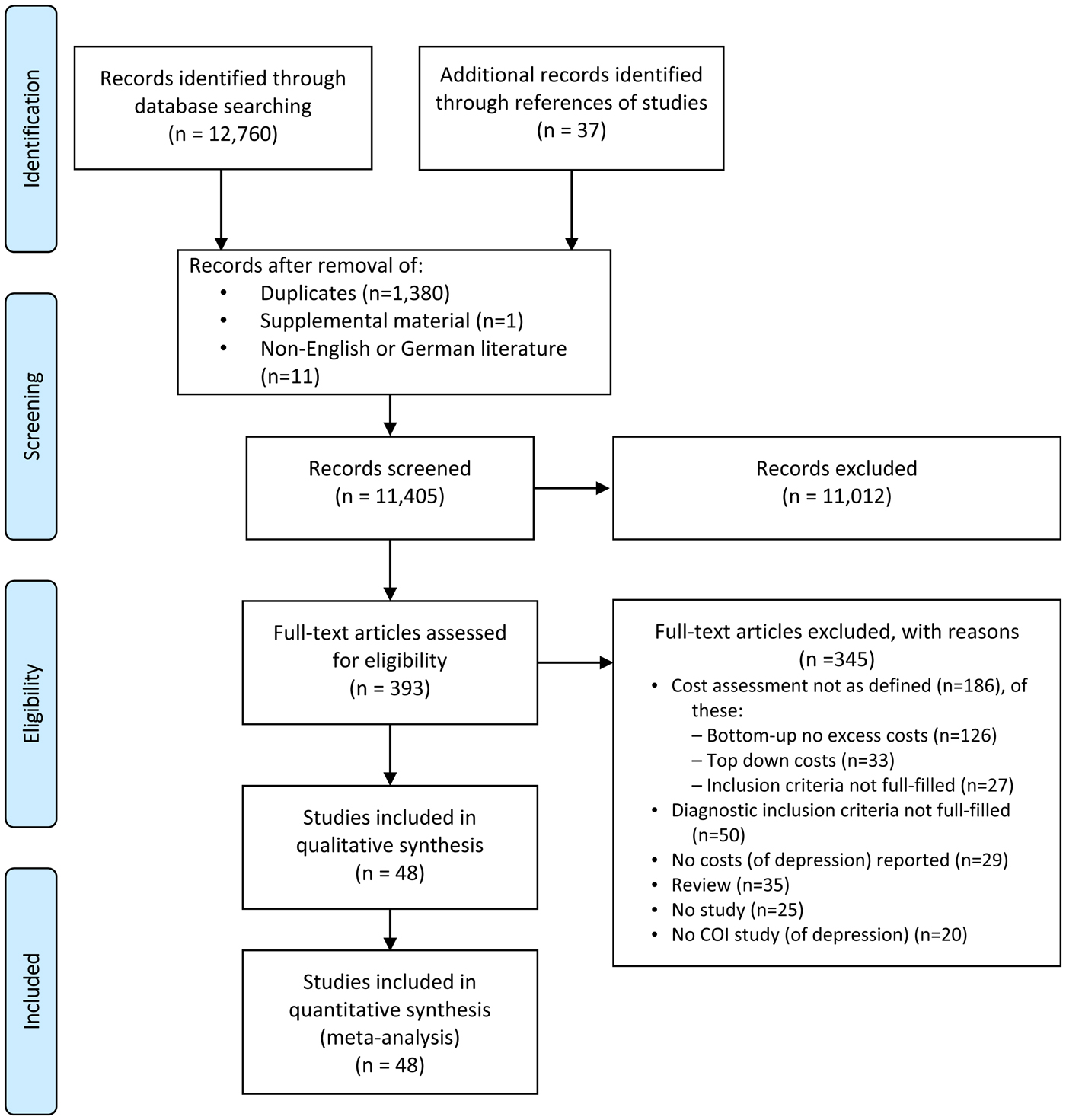
Fig. 1. PRISMA 2009 Flow diagram.
A total of 20 studies compared excess costs of depression in adults (D v. ND) (Simon et al., Reference Simon, VonKorff and Barlow1995; Druss et al., Reference Druss, Rosenheck and Sledge2000; Garis and Farmer, Reference Garis and Farmer2002; Carta et al., Reference Carta, Hardoy, Kovess, Dell'Osso and Carpiniello2003; Trivedi et al., Reference Trivedi, Lawrence, Blake, Rappaport and Feldhaus2004; Shvartzman et al., Reference Shvartzman, Weiner, Vardy, Friger, Sherf and Biderman2005; Thomas et al., Reference Thomas, Waxmonsky, Gabow, Flanders-McGinnis, Socherman and Rost2005; Gameroff and Olfson, Reference Gameroff and Olfson2006; Arnow et al., Reference Arnow, Blasey, Lee, Fireman, Hunkeler, Dea, Robinson and Hayward2009; Bosmans et al., Reference Bosmans, de Bruijne, de Boer, van Hout, van Steenwijk and van Tulder2010; Hamre et al., Reference Hamre, Witt, Glockmann, Ziegler, Kienle, Willich and Kiene2010; Stamm et al., Reference Stamm, Reinhard and Salize2010 , Woo et al., Reference Woo, Kim, Hwang, Frick, Choi, Seo, Kang, Kim, Ham, Lee and Park2011; Carstensen et al., Reference Carstensen, Andersson, Andre, Engstrom, Magnusson and Borgquist2012; Brilleman et al., Reference Brilleman, Purdy, Salisbury, Windmeijer, Gravelle and Hollinghurst2013; McTernan et al., Reference McTernan, Dollard and LaMontagne2013; Choi et al., Reference Choi, Lee, Matejkowski and Baek2014; Greenberg et al., Reference Greenberg, Fournier, Sisitsky, Pike and Kessler2015; Chiu et al., Reference Chiu, Lebenbaum, Cheng, de Oliveira and Kurdyak2017; Hsieh and Qin, Reference Hsieh and Qin2018), 12 studies reported excess depression costs in old age (D-Elderly v. ND-Elderly) (Callahan et al., Reference Callahan, Hui, Nienaber, Musick and Tierney1994; Callahan et al., Reference Callahan, Kesterson and Tierney1997; Fischer et al., Reference Fischer, Wei, Rolnick, Jackson, Rush, Garrard, Nitz and Luepke2002; Katon et al., Reference Katon, Lin, Russo and Unutzer2003; Luppa et al., Reference Luppa, Heinrich, Matschinger, Sandholzer, Angermeyer, König and Riedel-Heller2008; Vasiliadis et al., Reference Vasiliadis, Dionne, Preville, Gentil, Berbiche and Latimer2013; Bock et al., Reference Bock, Luppa, Brettschneider, Riedel-Heller, Bickel, Fuchs, Gensichen, Maier, Mergenthal, Schäfer, Schön, Weyerer, Wiese, van den Bussche, Scherer and König2014; Choi et al., Reference Choi, Lee, Matejkowski and Baek2014; Prina et al., Reference Prina, Huisman, Yeap, Hankey, Flicker, Brayne and Almeida2014; Alexandre et al., Reference Alexandre, Hwang, Roth, Gallo and Eaton2016; Bock et al., Reference Bock, Brettschneider, Weyerer, Werle, Wagner, Maier, Scherer, Kaduszkiewicz, Wiese, Moor, Stein, Riedel-Heller and König2016; Ludvigsson et al., Reference Ludvigsson, Bernfort, Marcusson, Wressle and Milberg2018) and two studies examined excess costs of depression in adolescents (D-Adolescents v. ND-Adolescents) (Guevara et al., Reference Guevara, Mandell, Rostain, Zhao and Hadley2003; Wright et al., Reference Wright, Katon, Ludman, McCauley, Oliver, Lindenbaum and Richardson2016). In total 16 studies compared comorbid depression excess costs among participants with a somatic disease (CD v. NCD) –predominantly diabetes, heart diseases, chronic pain – or after birth (Engel et al., Reference Engel, von Korff and Katon1996; Frasure-Smith et al., Reference Frasure-Smith, Lesperance, Gravel, Masson, Juneau, Talajic and Bourassa2000; Egede et al., Reference Egede, Zheng and Simpson2002; Petrou et al., Reference Petrou, Cooper, Murray and Davidson2002; Rosenzweig et al., Reference Rosenzweig, Weinger, Poirier-Solomon and Rushton2002; Sullivan et al., Reference Sullivan, Simon, Spertus and Russo2002; Finkelstein et al., Reference Finkelstein, Bray, Chen, Larson, Miller, Tompkins, Keme and Manderscheid2003; Gilmer et al., Reference Gilmer, O'Connor, Rush, Crain, Whitebird, Hanson and Solberg2005; Williams et al., Reference Williams, Narciso, Browne, Roberts, Weir and Gafni2005; Morgan et al., Reference Morgan, Byrne, Hughes, Petersen, Taylor, Robinson-Whelen, Hasche and Nosek2008; Arnow et al., Reference Arnow, Blasey, Lee, Fireman, Hunkeler, Dea, Robinson and Hayward2009; Rutledge et al., Reference Rutledge, Vaccarino, Johnson, Bittner, Olson, Linke, Cornell, Eteiba, Sheps, Francis, Krantz, Bairey Merz, Parashar, Handberg, Vido and Shaw2009; Edoka et al., Reference Edoka, Petrou and Ramchandani2011; Dagher et al., Reference Dagher, McGovern, Dowd and Gjerdingen2012; Rayner et al., Reference Rayner, Hotopf, Petkova, Matcham, Simpson and McCracken2016; Adam et al., Reference Adam, Flahiff, Kamble, Telen, Reed and De Castro2017).
A total of 30 studies were conducted in the region of the Americas, 14 in the European region and four in the Western Pacific region. The studies were published from the year 2000 onwards, with four exceptions (Callahan et al., Reference Callahan, Hui, Nienaber, Musick and Tierney1994; Simon et al., Reference Simon, VonKorff and Barlow1995; Engel et al., Reference Engel, von Korff and Katon1996; Callahan et al., Reference Callahan, Kesterson and Tierney1997). In total, 55 898 depressed and 674 414 non-depressed participants were encompassed by the studies, whereas study samples and sample sizes varied widely. Depression status was either assessed with disease-specific instruments or retrieved from medical diagnoses. For details, see Table 1.
Table 1. General characteristics
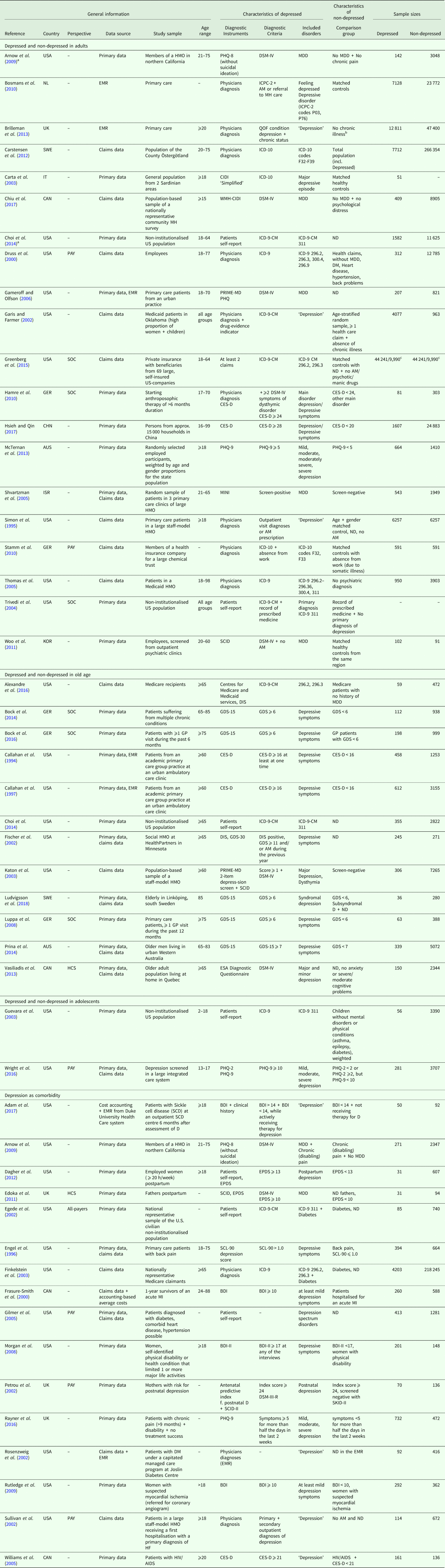
AM, Antidepressant Medication; BDI, BDI-II, Beck Depression Inventory; BL, Baseline; CES-D, Centre for Epidemiological Studies Depression Scale, German version; CAD, Coronary Artery Disease; CHD, Coronary Heart Disease; CIDI, CIDI-SF, Composite International Diagnostic Interview, short-form; CRC, Colorectal Cancer; DIS, Diagnostic Interview schedule; DM, Diabetes Mellitus; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition; EMR, Electronic Medical Record; EPDS, Edinburgh Postnatal Depression Scale; ESA, Étude sur la Santé des Ainés; FUP, Follow-up; GDS, GDS-15, Geriatric Depression Scale, 30-item-scale, 15-item-scale; GP, General Practitioner; HCC, Health Conditions Checklist; HCS, Healthcare System Perspective; HF, Heart Failure; HMO, Health Maintenance Organization; ICD-9, ICD-10, International Statistical Classification of Diseases and Related Health Problems, 9th revision, 10th revision; ICPC-2, International Classification of Primary Care, 2nd edition; MDD, Major Depressive Disorder; MDI, Major Depression Inventory; MH, Mental Health; MI, Myocardial Infarction; MINI, Mini-International Neuropsychiatric Interview; ND, No depression; PAY, Payer Perspective; PHQ-2, PHQ-9, Patient Health Questionnaire, 2 item depression screener, 9 item depression scale; PRIME-MD, Primary Care Evaluation of Mental Disorders; QOF, Quality and Outcomes Framework; SCID, Structured Clinical Interview for DSM-IV; SOC, Societal Perspective; WMH-CIDI, World Mental Health Composite International Diagnostic Interview.
a Reports also data in another patient subgroup.
b None of the included 17 chronic conditions incentivised within QOF.
c Sample sizes of direct/indirect costs.
Since we only included studies reporting excess costs from a bottom-up approach, cost assessment was based on the individual resource utilization per participant. The main data source was the primary data. Alternative data sources were claims data from healthcare providers, physician's electronic medical records or a combination of those. Since Hamre et al. (Reference Hamre, Witt, Glockmann, Ziegler, Kienle, Willich and Kiene2010) assessed costs after an intervention, we used the excess costs reported for the pre-study year. Table 2 provides details on cost assessment (cost categories reported and total costs). Time interval for costs was mostly 12 months, except for eight studies with time intervals <12 months (Callahan et al., Reference Callahan, Hui, Nienaber, Musick and Tierney1994; Katon et al., Reference Katon, Lin, Russo and Unutzer2003; Dagher et al., Reference Dagher, McGovern, Dowd and Gjerdingen2012; Bock et al., Reference Bock, Luppa, Brettschneider, Riedel-Heller, Bickel, Fuchs, Gensichen, Maier, Mergenthal, Schäfer, Schön, Weyerer, Wiese, van den Bussche, Scherer and König2014; Bock et al., Reference Bock, Brettschneider, Weyerer, Werle, Wagner, Maier, Scherer, Kaduszkiewicz, Wiese, Moor, Stein, Riedel-Heller and König2016; Rayner et al., Reference Rayner, Hotopf, Petkova, Matcham, Simpson and McCracken2016; Adam et al., Reference Adam, Flahiff, Kamble, Telen, Reed and De Castro2017; Ludvigsson et al., Reference Ludvigsson, Bernfort, Marcusson, Wressle and Milberg2018) and seven studies with time intervals >12 months (Petrou et al., Reference Petrou, Cooper, Murray and Davidson2002; Gilmer et al., Reference Gilmer, O'Connor, Rush, Crain, Whitebird, Hanson and Solberg2005; Rutledge et al., Reference Rutledge, Vaccarino, Johnson, Bittner, Olson, Linke, Cornell, Eteiba, Sheps, Francis, Krantz, Bairey Merz, Parashar, Handberg, Vido and Shaw2009; Bosmans et al., Reference Bosmans, de Bruijne, de Boer, van Hout, van Steenwijk and van Tulder2010; Carstensen et al., Reference Carstensen, Andersson, Andre, Engstrom, Magnusson and Borgquist2012; Prina et al., Reference Prina, Huisman, Yeap, Hankey, Flicker, Brayne and Almeida2014; Alexandre et al., Reference Alexandre, Hwang, Roth, Gallo and Eaton2016). Year of pricing had to be assumed for 15 studies (Callahan et al., Reference Callahan, Hui, Nienaber, Musick and Tierney1994; Simon et al., Reference Simon, VonKorff and Barlow1995; Engel et al., Reference Engel, von Korff and Katon1996; Callahan et al., Reference Callahan, Kesterson and Tierney1997; Frasure-Smith et al., Reference Frasure-Smith, Lesperance, Gravel, Masson, Juneau, Talajic and Bourassa2000; Rosenzweig et al., Reference Rosenzweig, Weinger, Poirier-Solomon and Rushton2002; Carta et al., Reference Carta, Hardoy, Kovess, Dell'Osso and Carpiniello2003; Katon et al., Reference Katon, Lin, Russo and Unutzer2003; Shvartzman et al., Reference Shvartzman, Weiner, Vardy, Friger, Sherf and Biderman2005; Williams et al., Reference Williams, Narciso, Browne, Roberts, Weir and Gafni2005; Gameroff and Olfson, Reference Gameroff and Olfson2006; Arnow et al., Reference Arnow, Blasey, Lee, Fireman, Hunkeler, Dea, Robinson and Hayward2009; Woo et al., Reference Woo, Kim, Hwang, Frick, Choi, Seo, Kang, Kim, Ham, Lee and Park2011; Prina et al., Reference Prina, Huisman, Yeap, Hankey, Flicker, Brayne and Almeida2014; Adam et al., Reference Adam, Flahiff, Kamble, Telen, Reed and De Castro2017).
Table 2. Cost assessment
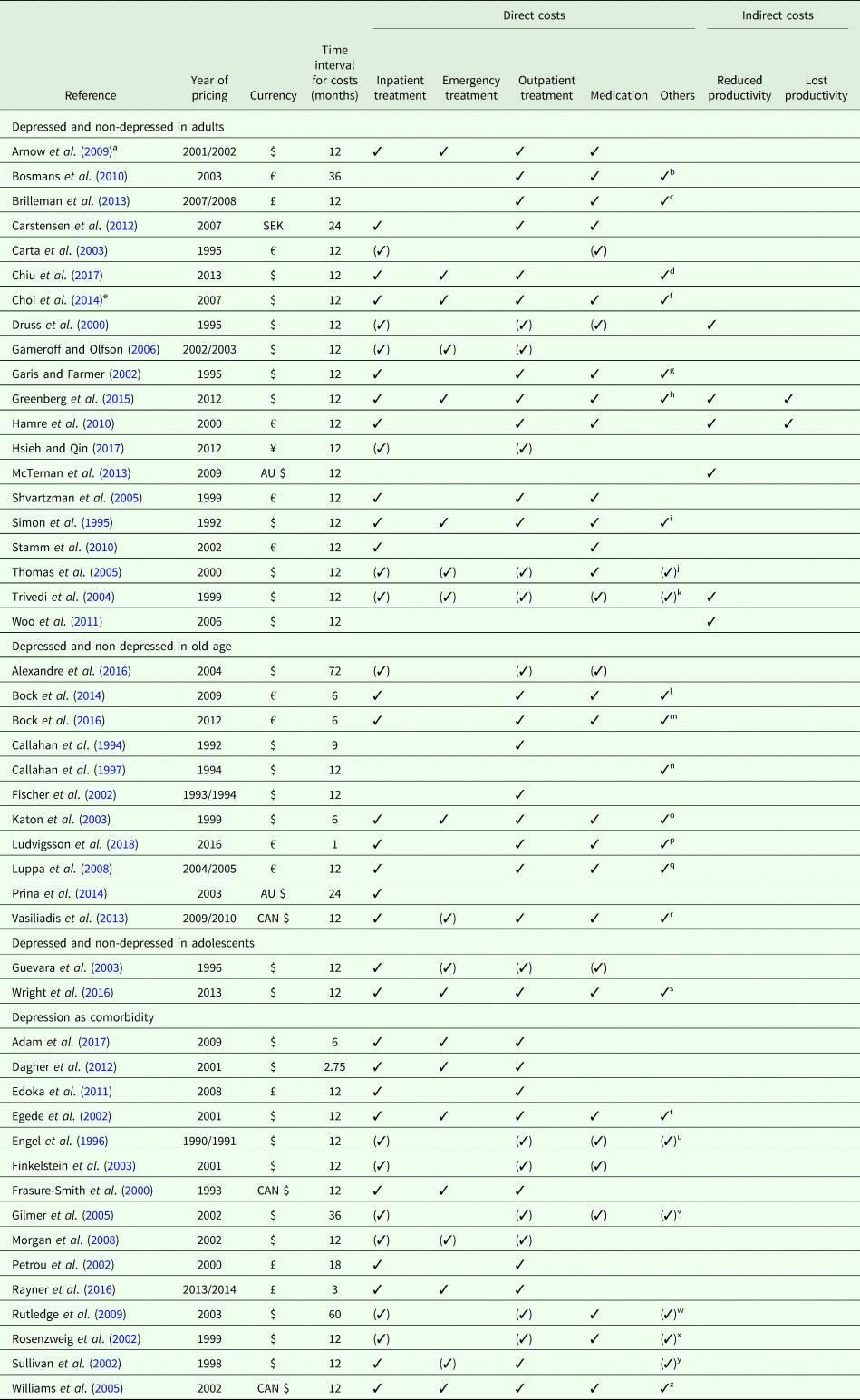
✓ Costs reported (✓) Costs considered in calculation of total costs, but not reported as single cost categories.
a Reports also data for depression as comorbidity.
b Costs reported: Dietician and Physical therapy (physiotherapy, cesar exercise therapy and mensendieck exercise therapy).
c Costs reported: Tests and investigations (standard surgery consultation, laboratory testing, GP requested hospital-based tests and investigations).
d Costs considered: Outpatient prescriptions for adults aged ⩾65, non-hospital residential care, ambulatory care, home care, medical devices.
e Reports also data for depressed and non-depressed in old age.
f Costs reported: Home health care and others.
g Costs reported: Home health/Medical supply and an all-other-costs category.
h Costs reported: Other medical services.
i Costs reported: Laboratory/Radiology.
j Costs considered: Diagnostic tests (laboratory and radiology).
k Costs considered: Home health and other medical equipment and services.
l Costs reported: Formal nursing care (Nursing home care, professional nursing care), informal care, medical supplies and dental prostheses.
m Costs reported: Nursing care (outpatient nursing care, domestic help, day care/short-term care, informal care).
n Costs reported: Diagnostic test charges (special procedures, diagnostic imaging, clinical pathology).
o Costs reported: Home health/Medical supply and an all-other-costs category.
p Costs reported: Non-pharmaceutical components, private health care.
q Costs reported: Medical supply and dentures, home care, assisted living, transportation, non-physician provider.
r Costs reported: Physicians fees (not included in any of the unit costs).
s Costs reported: Diagnostic tests (laboratory and radiology).
t Costs reported: Other medical expenditures (vision aids and other medical equipment and services).
u Costs considered: Radiology costs.
v Costs considered: Medical supply.
w Costs considered: Out-of-pocket for medical devices and alternative therapies, travel costs.
x Costs considered: Diagnostic tests (laboratory and radiology) and transportation.
y Costs considered: Long-term care costs, ambulance, home equipment costs.
z Costs reported: Food banks, house cleaning, outpatient laboratory test, all other, OOP cost.
Overall, 82% of the items in the quality assessment were fulfilled, while most studies lagged reporting perspective, sensitivity analysis and information about missing data. Detailed results of the quality assessment are shown in online Supplementary material S1. For nine studies, s.d. was calculated based on 95% CI or s.e. 11 studies did not state measures of variation and one study only reported s.d. for total excess costs. Summary data on mean annual excess costs (in 2017 US$-PPP) are provided in online Supplementary material S2. Results of meta-analyses are shown numerically and graphically as forest plots (Figs 2, 3 and online Supplementary material S3).
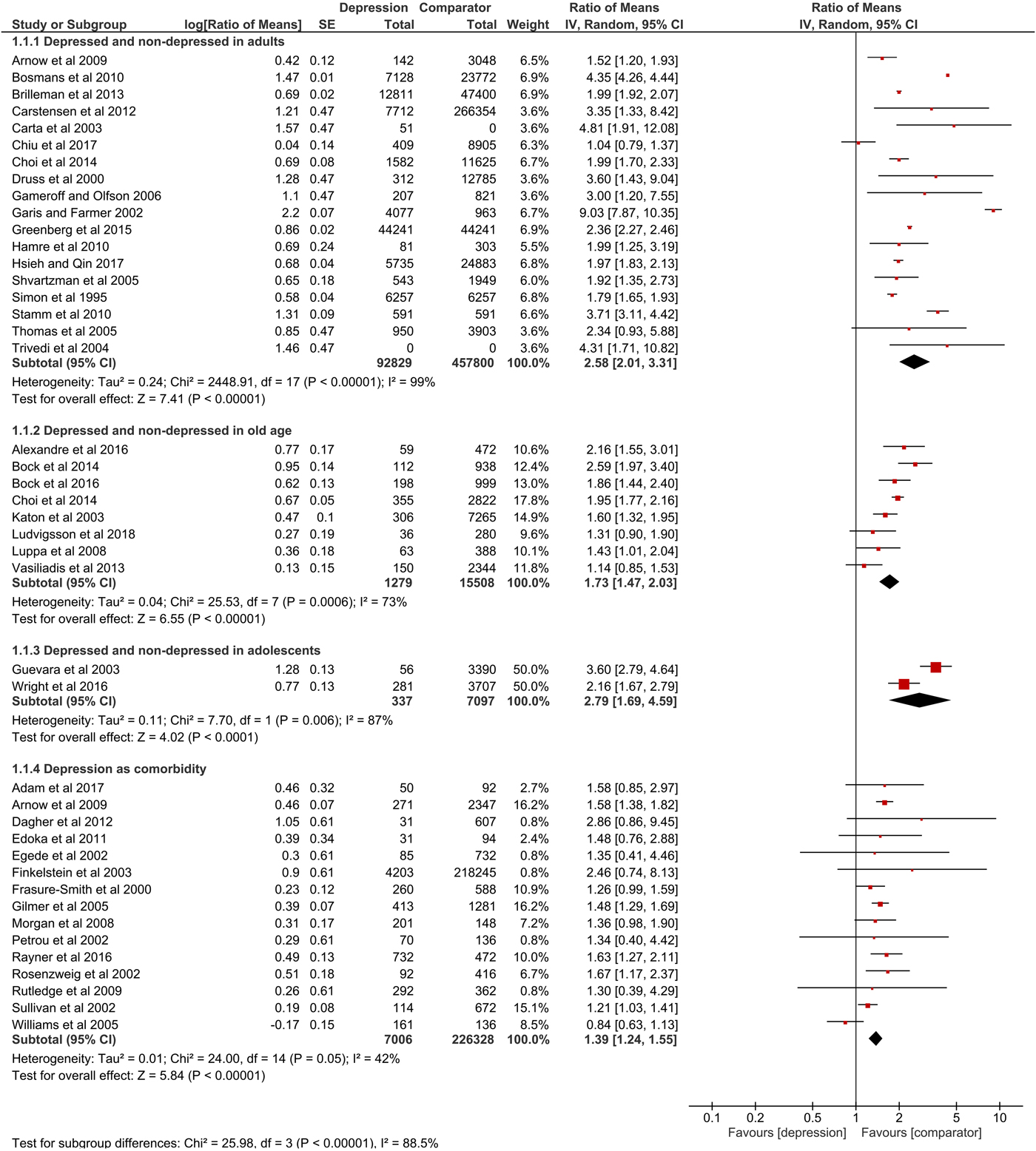
Fig. 2. Forest plot of total direct excess costs (Ratio of means, 95% CI).
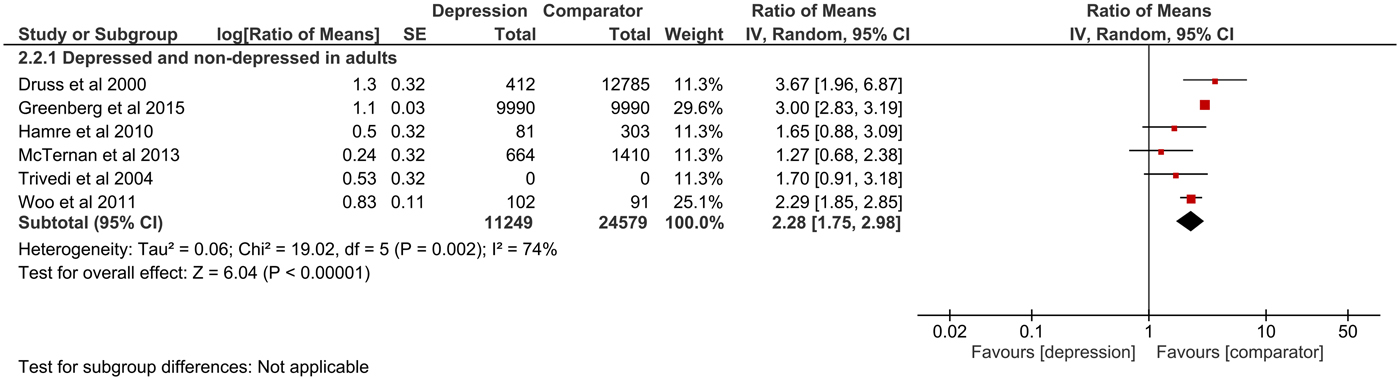
Fig. 3. Forest plot of total indirect excess costs (Ratio of means, 95% CI).
Total direct excess costs of depression ranged between $124 and 18 174 in the adults subgroup, between $358 and 14 225 in the elderly subgroup, between $2868 and 2883 in the adolescents subgroup and between $239 and 20 768 in the comorbidity subgroup. Meta-analysis of total direct excess costs was performed with all but seven studies that focused on singular cost categories (Callahan et al., Reference Callahan, Hui, Nienaber, Musick and Tierney1994; Engel et al., Reference Engel, von Korff and Katon1996; Callahan et al., Reference Callahan, Kesterson and Tierney1997; Fischer et al., Reference Fischer, Wei, Rolnick, Jackson, Rush, Garrard, Nitz and Luepke2002; Woo et al., Reference Woo, Kim, Hwang, Frick, Choi, Seo, Kang, Kim, Ham, Lee and Park2011; McTernan et al., Reference McTernan, Dollard and LaMontagne2013; Prina et al., Reference Prina, Huisman, Yeap, Hankey, Flicker, Brayne and Almeida2014). Depression was associated with significantly higher total direct excess costs in all subgroups. Expressed as point estimate [95% CI], total direct excess costs were higher for depressed v. non-depressed adults (2.58 [2.01–3.31], p < 0.0001, I 2 = 99%), depression in old age (1.73 [1.47–2.03], p < 0.0001, I 2 = 73%), depression in adolescents (2.79 [1.69–4.59], p < 0.0001, I 2 = 87%) and depression as comorbidity (1.39 [1.24–1.55], p < 0.0001, I 2 = 42%). Total indirect excess costs ranged between $153 and 12 374 in the D v. ND subgroup. Meta-analysis was performed with six studies and revealed higher excess costs for D v. ND (2.28 [1.75–2.98], p < 0.0001, I 2 = 74%) (Druss et al., Reference Druss, Rosenheck and Sledge2000; Trivedi et al., Reference Trivedi, Lawrence, Blake, Rappaport and Feldhaus2004; Hamre et al., Reference Hamre, Witt, Glockmann, Ziegler, Kienle, Willich and Kiene2010 , Woo et al., Reference Woo, Kim, Hwang, Frick, Choi, Seo, Kang, Kim, Ham, Lee and Park2011; McTernan et al., Reference McTernan, Dollard and LaMontagne2013; Greenberg et al., Reference Greenberg, Fournier, Sisitsky, Pike and Kessler2015).
Pooled results of 26 studies showed significantly higher outpatient excess costs for D v. ND (1.85 [1.64–2.10], p < 0.0001, I 2 = 91%), D-Elderly v. ND-Elderly (1.36 [1.18–1.57], p < 0.0001, I 2 = 55%) and CD v. NCD (1.35 [1.21–1.50], p < 0.0001, I 2 = 43%). We included 20 studies in the meta-analysis of medication costs. The pooled results showed significantly higher excess costs for D v. ND (2.89 [2.16–3.86], p < 0.0001, I 2 = 99%), D-Elderly v. ND-Elderly (1.47 [1.24–1.75], p < 0.0001, I 2 = 77%), CD v. NCD (1.35 [1.04–1.75], p = 0.02, I 2 = 94%). Meta-analysis of inpatient costs was conducted with 26 studies. Excess costs were significantly higher for D v. ND (2.82 [1.94–4.08], p < 0.0001, I 2 = 89%), D-Elderly v. ND-Elderly (1.92 [1.63–2.26], p < 0.0001, I 2 = 35%), D-Adolescents v. ND-Adolescents (4.10 [2.29–7.33], p < 0.0001, I 2 = 0%) and CD v. NCD (1.44 [1.09–1.90], p = 0.01, I 2 = 25%). Meta-analysis of emergency costs was performed with ten studies and revealed significant higher RoM for D v. ND (1.88 [1.49–2.37], p < 0.0001, I 2 = 90%), D-Elderly v. ND-Elderly (1.71 [1.36–2.16], p < 0.0001, I 2 = 0%) and CD v. NCD (1.62 [1.27–2.08], p = 0.0001, I 2 = 53%). Meta-analysis of other direct costs was conducted with 16 studies, with significantly higher excess costs for D v. ND (2.31 [1.65–3.24], p < 0.0001, I 2 = 98%) and D-Elderly v. ND-Elderly (1.75 [1.32–2.31], p < 0.0001, I 2 = 69%). Results for CD v. NCD were not significant (1.14 [0.88–1.49], p = 0.32, I 2 = 64%).
Heterogeneity in direct costs was high for all patient subgroups. Cost data are very sensitive to different framework conditions and settings (e.g. health systems, local prices or target populations), which results in heterogeneity between study results. We tried to cope with this problem using RoM as effect measure, but since costs have high variation by nature, wide statistical variation is to some extent reasonable. Meta-analysis showed that inpatient excess costs for the CD v. NCD subgroup scattered close to zero, which is comprehensible since hospitalisation is presumably caused by the primary disease being present in both groups. RoM of the study by Hamre et al. (Reference Hamre, Witt, Glockmann, Ziegler, Kienle, Willich and Kiene2010) were lower since excess costs were assessed in an anthroposophic setting with alternative therapies. Hence, fewer patients received antidepressant medication or psychotherapy.
Nevertheless, some studies showed considerable deviations whose impact was explored in a sensitivity analysis by excluding the studies as described below. Bosmans et al. (Reference Bosmans, de Bruijne, de Boer, van Hout, van Steenwijk and van Tulder2010) limited the depression group to participants with a prescription for antidepressants or a referral to mental health care and compared those to matched controls that did not meet the criteria, with the effect that RoM in outpatient and medication costs were considerably high. Luppa et al. (Reference Luppa, Heinrich, Matschinger, Sandholzer, Angermeyer, König and Riedel-Heller2008) had a lower RoM in outpatient costs due to high outliers in the comparison group. The analysis of Dagher et al. (Reference Dagher, McGovern, Dowd and Gjerdingen2012) was based on very small sample sizes, especially in inpatient and emergency costs, leading to high excess costs in these categories. RoM of medication costs among HIV/AIDS patients reported by Williams et al. (Reference Williams, Narciso, Browne, Roberts, Weir and Gafni2005) were extremely low. As depressed patients are found to be less compliant with medication recommendations, fewer participants have taken their HIV/AIDS medication resulting in lower excess costs (DiMatteo et al., Reference DiMatteo, Lepper and Croghan2000). Two studies were removed completely in the sensitivity analysis, because they differed extremely from other studies in length of study time or comparison group, affecting all cost categories: Chiu et al. (Reference Chiu, Lebenbaum, Cheng, de Oliveira and Kurdyak2017) had extremely lower RoM in all direct cost categories compared to other studies, which could be caused by an outstanding median study time of 10.6 years. RoM of Garis and Farmer, (Reference Garis and Farmer2002) had higher results in all reported direct cost categories, except for outpatient excess costs. Possible reasons could be an oversampling of young participants combined with a benefit limit for patients over 21 years and the exclusion of chronic illness in the comparison group.
Sensitivity analysis did not reveal significant changes in RoM and heterogeneity (I 2) for total direct costs, inpatient, medication and other direct costs. Detaching outliers reduced heterogeneity in outpatient costs for D-Elderly v. ND-Elderly (1.47 [1.36–1.58], p < 0.0001, I 2 = 0%). Heterogeneity in emergency costs decreased for D v. ND (2.17 [1.94–2.43], p < 0.0001, I 2 = 47%) and for CD v. NCD (1.57 [1.37–1.80], p < 0.0001, I 2 = 4%).
In a second sensitivity analysis, we removed articles in the German language in order to explore whether the inclusion of only one other language besides English biases the results. Only one study (Stamm et al., Reference Stamm, Reinhard and Salize2010) was removed and did not reveal significant changes in results. For more details, see online Supplementary material S4.
Discussion
The purpose of this study was to provide a structured overview of the current state of the literature of bottom-up COI-studies of depression with comparison group and to assess the impact of depression on costs. To our knowledge, this study is the first global systematic review combining study results on excess depression costs quantitatively in a meta-analytic framework. We found significantly higher excess depression costs for total direct and indirect costs and all cost categories except for other costs, although with considerable heterogeneity (I 2) in direct costs. Pooled RoM of total direct costs of depressed v. non-depressed were 179% higher in adolescents, 158% higher in adults and 73% higher in old age. In depression as comorbidity, pooled RoM of total direct costs was 39% higher. Pooled RoM of total indirect costs of depressed v. non-depressed was 128% higher in adults. Meta-analyses in the patient subgroups revealed that RoM decreased with age. As compared to the patient subgroups with participants from different age groups, RoM of comorbid depression was much lower.
The highest levels of RoM in adolescence could have been caused as a result of more resource-intensive treatment of mental disorders at a young age. Nevertheless, calculations were based on only two studies, which is why no generalizable conclusions should be drawn. Another explanation for a decreasing tendency with age could be that comorbidities increase with age, resulting in lower relative excess costs between depressed and non-depressed (Fortin et al., Reference Fortin, Bravo, Hudon, Vanasse and Lapointe2005; Schäfer et al., Reference Schäfer, Hansen, Schön, Höfels, Altiner, Dahlhaus, Gensichen, Riedel-Heller, Weyerer, Blank, König, von dem Knesebeck, Wegscheider, Scherer, van den Bussche and Wiese2012). This would also explain, why RoM in the comorbid depression subgroup was lower as compared to the subgroups with participants from different age groups. Results showed that comorbid depression increased costs, but transferability of results to a specific comorbid disease would need further investigations, since we did not distinguish between the main diseases.
Compared to the findings of preceding reviews (Luppa et al., Reference Luppa, Heinrich, Angermeyer, König and Riedel-Heller2007; Mrazek et al., Reference Mrazek, Hornberger, Altar and Degtiar2014), this study did not only reveal a positive association between depression and excess costs, but also allows to make precise statements about the amount of excess. In the old age patient subgroup, the review of Luppa et al. (Reference Luppa, Sikorski, Motzek, Konnopka, König and Riedel-Heller2012) also found higher total costs of depressed compared to non-depressed, but of a smaller magnitude than in our findings. A possible explanation could be that only studies with comparable study design were included, resulting in three studies. By contrast, results regarding outpatient excess costs matched with our findings. We found increased excess costs of depression as a comorbidity of other somatic diseases, whereas foregoing reviews of depression as comorbidity found varying results. Another important difference was, that these reviews included also bipolar disorders. Lehnert et al.( Reference Lehnert, Konnopka, Riedel-Heller and König2011) and Molosankwe et al. (Reference Molosankwe, Patel, José Gagliardino, Knapp and McDaid2012) found that coexistent depression increased costs of treating diabetes. Sambamoorthi et al. (Reference Sambamoorthi, Shah and Zhao2017) found an increase in costs of treating arthritis when depression coexisted. Baumeister et al. (Reference Baumeister, Knecht and Hutter2012) found higher direct, but not indirect costs in the treatment of chronic back pain with comorbid depression.
There might be various reasons for high heterogeneity. First, data originated from 11 different countries of studies published between 1994 and 2018. Second, included studies comprised different degrees of depression severity. On one side, the inclusion of mild depression allows to consider the whole disease pattern. Otherwise, different degrees of severity could have caused variability in results. Additionally, diagnostic instruments, data sources, target populations and sample sizes varied between studies. Furthermore, differences in cost assessment of studies could have caused heterogeneity. Adjusted and unadjusted excess costs were included in our analysis, a potential influencing factor for variability in results. Moreover, in direct costs, included services and monetary valuation were diverse. Since excess costs reported by studies were split according to predefined direct cost categories with one additional category including all other direct costs, heterogeneity in the other direct cost category was to be expected. Indirect excess costs were assessed only in the D v. ND patient subgroup. Mainly excess costs of reduced productivity (sickness absence, costs of presenteeism) were assessed, resulting in more homogeneity across studies compared to direct excess costs.
This systematic review and meta-analysis of COI-studies of depression were unlimited with respect to region and year of publication, resulting in a sufficiently large number of eligible studies. Another strength of our study was that the literature search and study selection was conducted independently by two reviewers. RoM as a new method for continuous outcomes achieved meaningful results, providing a useful tool for meta-analyses with cost data. When interpreting our results, several limitations should be considered. Overall methodological quality was good, but shortcomings manifested in reporting perspective, missing data and sensitivity analysis. We tried to include all eligible articles in this study and imputed missed s.e. to reduce selection bias. However, studies were restricted to English or German language and bottom-up studies, a potential source of reporting bias. In addition, bottom-up studies tend to involve small sample sizes and more serious cases, leading to an overestimation of excess costs at population-level. Otherwise, few studies assessed indirect excess costs and none quantified costs of reduced productivity due to mortality, although depression is associated with high suicidal risk, which may have underestimated indirect excess costs. Since we focused on studies reporting excess costs of depression, true disease-specific costs were presented, though a large number of COI-studies without a comparison group were ineligible for this study.
In summary, these findings highlight the burden of depression at all ages and as a comorbidity. As a result, screening and prevention programs should be offered for broader target groups. More assessment of indirect costs and methodological uniformity would be highly desirable for future COI research in depression.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796019000180
Author ORCIDs
Hannah König, 0000-0002-4515-8405.
Acknowledgements
None.
Financial support
This work was supported by the Innovation Committee at the Federal Joint Committee (G-BA) in Germany (grant number 01NVF16018).
Conflict of interest
None.
Ethical standards
Not applicable.
Availability of data and materials
The data supporting the findings of our study cannot be provided online, since the articles included in our systematic review and meta-analysis are protected by copyright. Additional data from our analysis can be requested by the corresponding author.






