Cannabidiol (CBD) represents the second most abundant phytocannabinoid in Cannabis plant after the psychoactive tetrahydrocannabinol (Δ9-THC) and, in recent years, an increasing body of evidences emphasised its promising role as a treatment in several medical conditions (Fasinu et al. Reference Fasinu, Phillips, ElSohly and Walker2016). Indeed, CBD exhibits various therapeutic effects that range from antioxidant, anti-inflammatory and neuroprotective (Watt & Karl, Reference Watt and Karl2017) to anticonvulsive (Perucca, Reference Perucca2017), anti-emetic (Parker et al. Reference Parker, Rock and Limebeer2011) and analgesic (Lötsch et al. Reference Lötsch, Weyer-Menkhoff and Tegeder2018).
Moreover, several animal studies and clinical trials on healthy controls suggested its potential role as an antipsychotic (Hahn, Reference Hahn2018), anxiolytic (Soares & Campos, Reference Soares and Campos2017) and anti-craving (Lee et al. Reference Lee, Bertoglio, Guimarães and Stevenson2017) drug, as well as a pro-cognitive (Osborne et al. Reference Osborne, Solowij and Weston-Green2017) and antidepressant (de Mello Schier et al. Reference de Mello Schier, de Oliveira Ribeiro, Coutinho, Machado, Arias-Carrión, Crippa, Zuardi, Nardi and Silva2014) compound. Therefore, it is not surprising that CBD has been considered as a new potential treatment in several psychiatric disorders.
In this regard, in order to link the so far hypothesised pharmacological mechanisms of action of CBD to its clinical effect in psychiatric disorders, we performed a bibliographic search in PubMed using ‘Cannabidiol AND Psychiatry’, ‘Cannabidiol AND Psychosis’, ‘Cannabidiol AND Schizophrenia’, ‘Cannabidiol AND Bipolar Disorder’, ‘Cannabidiol AND Depression’, ‘Cannabidiol AND Anxiety’, ‘Cannabidiol AND Substance Use Disorder’, ‘Cannabidiol AND Withdrawal Syndrome’ as key words. Additional articles were identified through the reference lists of the papers. We selected published studies from January 1995 until April 2018. We excluded both pre-clinical studies and clinical trials in healthy controls. Fourteen studies were finally included (five case reports, one open-label pilot study, one double-blinded controlled clinical trial, seven randomised double-blinded controlled clinical trials), matching methods and results summarised in Table 1.
Table 1. Summary of the studies described in the manuscript
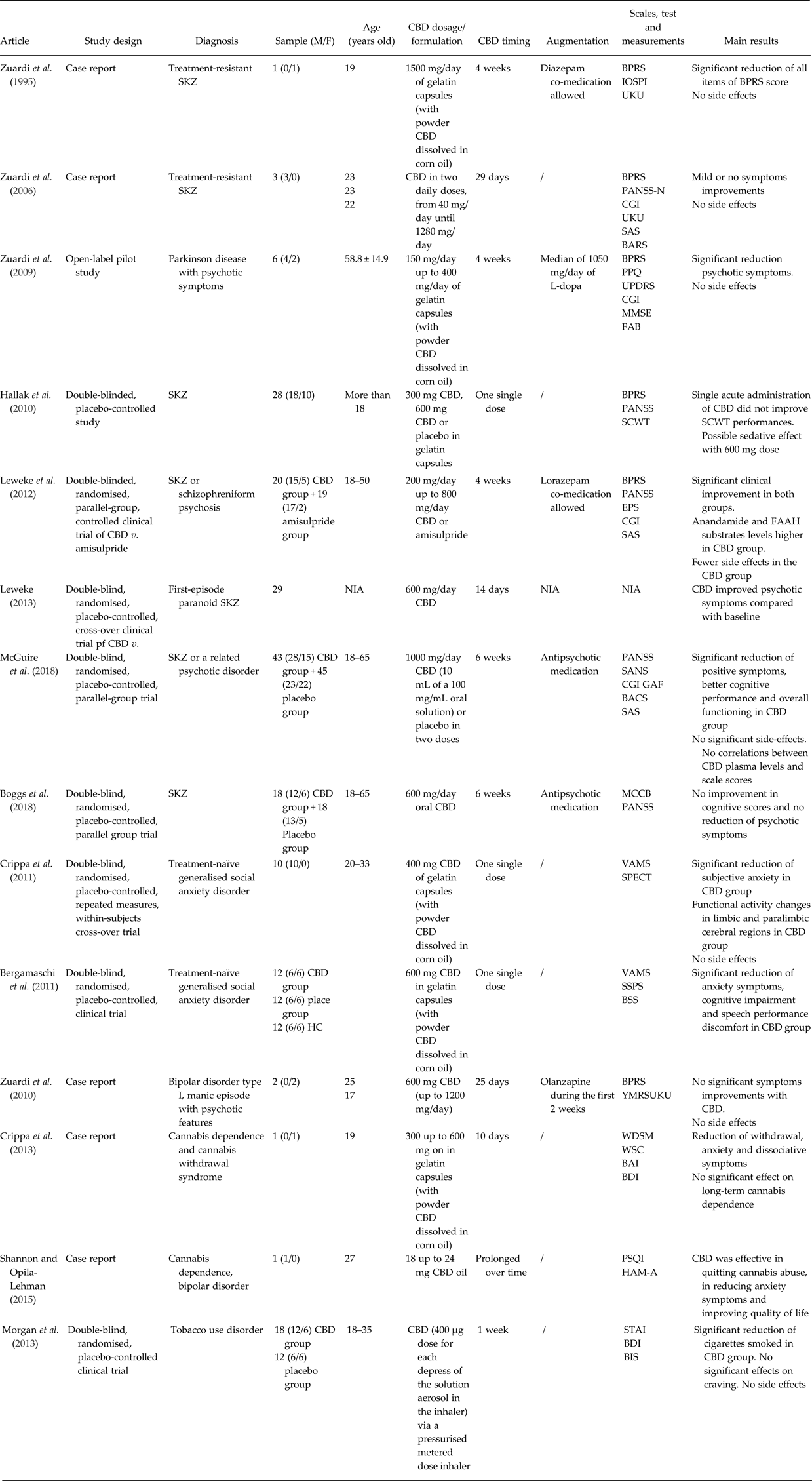
BACS, Brief Assessment of Cognition in Schizophrenia; BAI, Beck Anxiety Inventory; BARS, Barnes Akathisia Rating Scale; BDI, Beck Depression Inventory; BIS, Behaviour Impulsivity Scale; BPRS, Brief Psychiatric Rating Scale; BSS, Bodily Symptoms Scale; CBD, cannabidiol; CGI, Global Clinical Impression Scale; EPS, Extrapyramidal Symptoms Rating Scale; FAAH, fatty acid amide hydrolase; FAB, Frontal Assessment Battery; GAF, Global Assessment of Functioning; Hamilton Anxiety Rating Scale; HC, healthy controls; IOPSI, Interactive Observation Scale for Psychiatric Inpatients; MCCB, Matrix Consensus Cognitive Battery; MMSE, Mini-Mental Status Examination; MWSC, Marijuana Withdrawal Symptom Checklist; NIA, no information available; PANSS-N, Negative Subscale of Positive and Negative Syndrome Scale; PPQ, Parkinson Psychosis Questionnaire; PSQI, Pittsburgh Sleep Quality Index; SANS, Scale for Assessment of Negative Symptoms; SAS, Social Anxiety Scale; SAS, Simpson-Angus Scale for Parkinson; SCWT, Stroop Color-Word Test; SKZ, schizophrenia; SPECT, single-photon emission computer tomography; SPSS, Self-Statements during Public Speaking Scale; STAI, Spielberger Trait Anxiety Inventory; UKU, UKU Side Effect Rating Scale; UPDRS, Unified Parkinson's Disease Rating Scale; VAMS, Visual Analogue Mood Scale; WDS, Withdrawal Discomfort Score; YMRS, Young Mania Rating Scale.
CBD and psychosis
Eight studies evaluated the clinical effect of CBD in psychosis. Five of these studies found a beneficial effect of CBD in reducing psychotic symptoms in schizophrenia (SKZ) (Zuardi et al. Reference Zuardi, Morais, Guimaraes and Mechoulam1995; Leweke et al. Reference Leweke, Piomelli, Pahlisch, Muhl, Gerth, Hoyer, Klosterkötter, Hellmich and Koethe2012; Leweke, Reference Leweke2013; McGuire et al. Reference McGuire, Robson, Cubala, Vasile, Morrison, Barron, Taylor and Wright2018) and in Parkinson's disease (Zuardi et al. Reference Zuardi, Crippa, Hallak, Pinto, Chagas, Rodrigues, Dursun and Tumas2009), while the others found only mild effects or no improvement over psychotic symptoms in patients with SKZ (Zuardi et al. Reference Zuardi, Hallak, Dursun, Morais, Sanches, Musty and Crippa2006; Boggs et al. Reference Boggs, Surti, Gupta, Gupta, Niciu and Pittman2018) or in bipolar disorder (BD) patients experiencing a manic episode with psychotic features (Zuardi et al. Reference Zuardi, Crippa, Dursun, Morais, Vilela, Sanches and Hallak2010).
The majority of studies attributed the potential antipsychotic properties of CBD to its ability to directly inhibit the reuptake of anandamide, an endocannabinoid that exhibits neurogenic and anti-inflammatory activity (Pisanti et al. Reference Pisanti, Malfitano, Ciaglia, Lamberti, Ranieri, Cuomo, Abate, Faggiana, Proto, Fiore, Laezza and Bifulco2017) and plays a major role in mood regulation, cognition and behaviour (Di Marzo & Petrosino, Reference Di Marzo and Petrosino2007). Moreover, CBD can also reduce endocannabinoids degradation by blocking fatty acid amide hydrolase function (Pisanti et al. Reference Pisanti, Malfitano, Ciaglia, Lamberti, Ranieri, Cuomo, Abate, Faggiana, Proto, Fiore, Laezza and Bifulco2017). Consequently, the increase of endocannabinoids, such as anandamide, in postsynaptic neurons may regulate presynaptic release of γ-aminobutyric acid and glutamate as well as stabilise dopamine neurotransmission (Gururajan & Malone, Reference Gururajan and Malone2016). Moreover, CBD has low affinity for cannabinoid receptor type 1 (CB1R) and type 2 (CB2R) with a not specific indirect antagonism when these receptors are activated by Δ9-THC (Pisanti et al. Reference Pisanti, Malfitano, Ciaglia, Lamberti, Ranieri, Cuomo, Abate, Faggiana, Proto, Fiore, Laezza and Bifulco2017). Therefore, CBD may likely contrast Δ9-THC psychotropic activity through a non-competitive negative allosteric modulation of CB1R by binding to a distinct allosteric site (Laprairie et al. Reference Laprairie, Bagher, Kelly and Denovan-Wright2015). Furthermore, while the antipsychotic effect of CBD has been mainly related to the enhanced endocannabinoids signalling, some authors recently hypothesised that CBD may exert its antipsychotic action through a partial agonist activity on dopamine D2 receptors, similarly to the atypical antipsychotic aripiprazole (Seeman, Reference Seeman2016), and through the activation of vanilloid receptor 1, a non-selective calcium channel, thus facilitating glutamate pre-synaptic release (Campos et al. Reference Campos, Moreira, Gomes, Del Bel and Guimaraes2012).
Interestingly, in the study carried out by Leweke et al. (Leweke et al. Reference Leweke, Piomelli, Pahlisch, Muhl, Gerth, Hoyer, Klosterkötter, Hellmich and Koethe2012), patients with SKZ were randomised to either amisulpride treatment or CBD administration for 28 days. The authors showed that both CBD and amisulpride groups had a significant reduction of psychotic symptoms and no difference in clinical efficacy was detected between the two different treatments. Moreover, the CBD group showed significantly higher serum level of both anandamide and fatty acid amide hydrolase substrates, a result which has been directly related to the CBD antipsychotic properties (Hahn, Reference Hahn2018). Finally, McGuire et al. (McGuire et al. Reference McGuire, Robson, Cubala, Vasile, Morrison, Barron, Taylor and Wright2018) detected a significant reduction of positive symptoms in patients with SKZ treated with CBD compared with placebo but the authors did not find any significant correlation between CBD plasma levels or its hydroxyl metabolites (6-OH-CBD, 7-OH-CBD) and the scores of scales assessing psychotic symptoms.
In conclusion, overall these evidences suggest that CBD may exert antipsychotic effects in patients with SKZ (Zuardi et al. Reference Zuardi, Morais, Guimaraes and Mechoulam1995; Leweke et al. Reference Leweke, Piomelli, Pahlisch, Muhl, Gerth, Hoyer, Klosterkötter, Hellmich and Koethe2012; Leweke, Reference Leweke2013; McGuire et al. Reference McGuire, Robson, Cubala, Vasile, Morrison, Barron, Taylor and Wright2018) and Parkinson's psychosis (Zuardi et al. Reference Zuardi, Crippa, Hallak, Pinto, Chagas, Rodrigues, Dursun and Tumas2009). However, in three studies, CBD was ineffective to treat psychotic symptoms in BD patients (Zuardi et al. Reference Zuardi, Crippa, Dursun, Morais, Vilela, Sanches and Hallak2010) and outpatients with SKZ (Zuardi et al. Reference Zuardi, Hallak, Dursun, Morais, Sanches, Musty and Crippa2006; Boggs et al. Reference Boggs, Surti, Gupta, Gupta, Niciu and Pittman2018). Moreover, the antipsychotic effect of CBD seemed to be related to the endocannabinoids plasma-level increase (Leweke et al. Reference Leweke, Piomelli, Pahlisch, Muhl, Gerth, Hoyer, Klosterkötter, Hellmich and Koethe2012), but not to CBD serum levels (McGuire et al. Reference McGuire, Robson, Cubala, Vasile, Morrison, Barron, Taylor and Wright2018). However, these results need further investigations. Therefore, since the facilitation of endocannabinoid signalling was the most likely hypothesised antipsychotic mechanism, the discrepancy of results among studies emphasised the need to evaluate endocannabinoid plasma-level changes in order to detect their potential contribution to the antipsychotic action.
CBD and anxiety disorder
The anxiolytic properties of CBD have been investigated by two clinical studies, carried out by the same research group, in patients diagnosed with generalised social anxiety disorder (SAD) (Bergamaschi et al. Reference Bergamaschi, Queiroz, Chagas, De Oliveira, De Martinis, Kapczinski, Quevedo, Roesler, Schröder, Nardi, Martín-Santos, Hallak, Zuardi and Crippa2011; Crippa et al. Reference Crippa, Nogueira Derenusson, Borduqui Ferrari, Wichert-Ana, Duran, Martin-Santos, Vinícius Simões, Bhattacharyya, Fusar-Poli, Atakan, Santos Filho, Freitas-Ferrari, McGuire, Zuardi, Busatto and Hallak2011). Interestingly, the authors showed that a single dose of CBD not only significantly decreased subjective anxiety symptoms (Crippa et al. Reference Crippa, Nogueira Derenusson, Borduqui Ferrari, Wichert-Ana, Duran, Martin-Santos, Vinícius Simões, Bhattacharyya, Fusar-Poli, Atakan, Santos Filho, Freitas-Ferrari, McGuire, Zuardi, Busatto and Hallak2011), but also reduced cognitive impairment, speech performance discomfort and alert in anticipatory speech during a Simulation Public Speaking Test (Bergamaschi et al. Reference Bergamaschi, Queiroz, Chagas, De Oliveira, De Martinis, Kapczinski, Quevedo, Roesler, Schröder, Nardi, Martín-Santos, Hallak, Zuardi and Crippa2011), in comparison to the placebo group.
In this regard, the anxiolytic effect exerted by CBD has been mainly related to its agonist activity towards serotonin type 1A (5HT1A) receptors (Soares & Campos, Reference Soares and Campos2017) in specific cerebral areas, including the dorsal periaqueductal grey, the bed nucleus of the stria terminalis and the medial prefrontal cortex (Campos et al. Reference Campos, Moreira, Gomes, Del Bel and Guimaraes2012). Specifically, even if the exact agonist mechanism is still unclear, some authors proposed that CBD may activate 5HT1A receptors by increasing guanosine-5′-triphosphate binding to Gi protein and by reducing cyclic adenosine monophosphate concentration, similarly to the serotonin neurotransmitter (Russo et al. Reference Russo, Burnett, Hall and Parker2005). Moreover, the acute anxiolytic effect of CBD has also been related to its capacity to modify cerebral blood flow in brain sites typically involved in anxiety, such as amygdala, hippocampus, hypothalamus and cingulate cortex (Soares & Campos, Reference Soares and Campos2017). In this perspective, Crippa et al. (Crippa et al. Reference Crippa, Nogueira Derenusson, Borduqui Ferrari, Wichert-Ana, Duran, Martin-Santos, Vinícius Simões, Bhattacharyya, Fusar-Poli, Atakan, Santos Filho, Freitas-Ferrari, McGuire, Zuardi, Busatto and Hallak2011) performed a cerebral single-photon emission computed tomography scan in ten patients with SAD before and after acute CBD or placebo administration. Interestingly, the authors showed that while a reduced regional cerebral blood flow in left parahippocampal gyrus, hippocampus and inferior temporal gyrus was detected among the patients taking CBD, an increased radiotracer uptake was found in the right posterior cingulate gyrus in the same group.
In conclusion, these findings suggest that, in patients with SAD, CBD may exert an acute anxiolytic effect when administered in a single high dose. Remarkably, this effect may be mainly related to the capacity of CBD to suddenly modify cerebral blood flow in limbic and paralimbic areas (Crippa et al. Reference Crippa, Nogueira Derenusson, Borduqui Ferrari, Wichert-Ana, Duran, Martin-Santos, Vinícius Simões, Bhattacharyya, Fusar-Poli, Atakan, Santos Filho, Freitas-Ferrari, McGuire, Zuardi, Busatto and Hallak2011) and to agonise 5HT1A receptors (Bergamaschi et al. Reference Bergamaschi, Queiroz, Chagas, De Oliveira, De Martinis, Kapczinski, Quevedo, Roesler, Schröder, Nardi, Martín-Santos, Hallak, Zuardi and Crippa2011).
CBD and substance abuse disorder
The pharmacological effects of CBD have also been tested in the treatment of substance abuse disorder by three studies. The case report carried out by Crippa et al. (Crippa et al. Reference Crippa, Hallak, Machado-De-Sousa, Queiroz, Bergamaschi, Chagas and Zuardi2013) found that a 10 days oral administration of CBD may acutely reduce withdrawal, anxiety and dissociative symptoms in a patient with cannabis dependence who previously failed to quit cannabis consumption due to serious abstinence symptoms. However, the authors showed that 10 days treatment with CBD was not effective to interrupt long-term relapses of cannabis abuse (Crippa et al. Reference Crippa, Hallak, Machado-De-Sousa, Queiroz, Bergamaschi, Chagas and Zuardi2013). In contrast, in a subsequent case report, Shannon and Opila-Lehman (Shannon & Opila-Lehman, Reference Shannon and Opila-Lehman2015) reported that a prolonged administration of CBD to one BD patient with cannabis dependence not only reduced anxiety symptoms and ameliorate overall quality of life, but also prevented the patient from resuming cannabis consumption. Finally, a potential role of CBD in the treatment of tobacco use disorder has been suggested by the only one double-blind randomised controlled clinical trial performed by Morgan et al., who showed that the CBD group significantly decreased the number of cigarettes smoked without, though, exhibiting any specific beneficial effects on craving symptoms, compared with the placebo group (Morgan et al. Reference Morgan, Das, Joye, Curran and Kamboj2013).
Overall these results might be explained considering that long-term cannabis consumption has been linked to a progressive desensitisation and downregulation of CB1R, which, in turn, may cause the onset of withdrawal symptoms when the substance is missing (Bonnet & Preuss, Reference Bonnet and Preuss2017). Therefore, the capacity of CBD to reduce substance-induced withdrawal symptoms has been attributed to its ability to acutely antagonise CB1R and to chronically enhance the physiological endocannabinoid neurotransmission (Prud'Homme et al. Reference Prud'Homme, Cata and Jutras-Aswad2015). With regards to tobacco addiction, the inhibition of acid amide hydrolase induced by CBD has been hypothesised to reduce the reinforcing effects of nicotine (Morgan et al. Reference Morgan, Das, Joye, Curran and Kamboj2013). Additionally, the agonist activity of CBD towards 5HT1A receptors may also contribute to the anti-craving effect of CBD and to reduce the relapses of substance abuse by regulating drug reward system, anxiety symptoms and stress management (Prud'Homme et al. Reference Prud'Homme, Cata and Jutras-Aswad2015). Finally, CBD may regulate the glutamatergic signalling through modulation of serotoninergic and endocannabinoid systems and this mechanism may also have a role in the treatment of addictive behaviours (Rodríguez-Muñoz et al. Reference Rodríguez-Muñoz, Sánchez-Blázquez, Merlos and Garzón-Niño2016), since a dysregulation of glutamatergic transmission has been widely related to both drug-seeking behaviours and abuse relapses (Prud'Homme et al. Reference Prud'Homme, Cata and Jutras-Aswad2015).
These evidences suggest that acute administration of CBD may reduce withdrawal symptoms of cannabis dependence (Crippa et al. Reference Crippa, Hallak, Machado-De-Sousa, Queiroz, Bergamaschi, Chagas and Zuardi2013), but the treatment needs to be prolonged over time in order to help quitting cannabis (Shannon & Opila-Lehman, Reference Shannon and Opila-Lehman2015) or tobacco use (Morgan et al. Reference Morgan, Das, Joye, Curran and Kamboj2013). The efficacy of CBD in the treatment of addictive disorders has been mostly hypothetically linked to the modulation of endocannabinoid, serotoninergic and glutamatergic systems.
CBD and cognitive impairment in psychiatric patients
As regards to neuropsychological functioning, three studies have considered the pro-cognitive effects of CBD in psychotic patients (Hallak et al. Reference Hallak, Machado-de-Sousa, Crippa, Sanches, Trzesniak, Chaves, Bernardo, Regalo and Zuardi2010; Boggs et al. Reference Boggs, Surti, Gupta, Gupta, Niciu and Pittman2018; McGuire et al. Reference McGuire, Robson, Cubala, Vasile, Morrison, Barron, Taylor and Wright2018). A single oral dose (Hallak et al. Reference Hallak, Machado-de-Sousa, Crippa, Sanches, Trzesniak, Chaves, Bernardo, Regalo and Zuardi2010) or a 6-week oral administration (Boggs et al. Reference Boggs, Surti, Gupta, Gupta, Niciu and Pittman2018) of CBD were not more effective than placebo in ameliorating cognitive performances among outpatients with SKZ. In contrast, McGuire et al. (McGuire et al. Reference McGuire, Robson, Cubala, Vasile, Morrison, Barron, Taylor and Wright2018) detected a trend of higher enhancement, although not statistically significant, in cognitive performance and overall functioning in patients with SKZ treated with oral CBD for 6 weeks in adjunction to their ongoing antipsychotic medications.
As a whole, these findings are not surprising, especially because preclinical studies attributed the potential role of CBD in the treatment of cognitive impairment to its capacity to reduce both inflammatory state and oxidative stress as well to promote neurogenesis by increasing brain-derived neurotrophic factor levels (Osborne et al. Reference Osborne, Solowij and Weston-Green2017). Finally, the activity of CBD towards 5HT1A and adenosine A2A receptors may also contribute to improve cognitive performances (Osborne et al. Reference Osborne, Solowij and Weston-Green2017).
Therefore, it may be speculated that CBD pro-cognitive effects may need longer time of administration in order to detect significant cognitive enhancement, since acute or long-term CBD administration within 6 weeks seemed to not significantly improve cognitive functioning in SKZ patients (Hallak et al. Reference Hallak, Machado-de-Sousa, Crippa, Sanches, Trzesniak, Chaves, Bernardo, Regalo and Zuardi2010; Boggs et al. Reference Boggs, Surti, Gupta, Gupta, Niciu and Pittman2018; McGuire et al. Reference McGuire, Robson, Cubala, Vasile, Morrison, Barron, Taylor and Wright2018).
Conclusions
In conclusion, the so far investigated mechanisms of action of CBD may likely explain the preliminary evidences of efficacy in the treatment of psychiatric disorders, especially in psychotic, anxiety and substance-induced disorders. However, the findings presented in this literature overview must be considered in light of some limitations. First, there are still few randomised placebo-controlled clinical trials with small sample size. Second, CBD was administered in inconsistent dosage, formulation and timing of administration. Third, some studies tested CBD efficacy in adjunction to standard medications (Zuardi et al. Reference Zuardi, Morais, Guimaraes and Mechoulam1995, Reference Zuardi, Crippa, Hallak, Pinto, Chagas, Rodrigues, Dursun and Tumas2009, Reference Zuardi, Crippa, Dursun, Morais, Vilela, Sanches and Hallak2010; Leweke et al. Reference Leweke, Piomelli, Pahlisch, Muhl, Gerth, Hoyer, Klosterkötter, Hellmich and Koethe2012; Boggs et al. Reference Boggs, Surti, Gupta, Gupta, Niciu and Pittman2018; McGuire et al. Reference McGuire, Robson, Cubala, Vasile, Morrison, Barron, Taylor and Wright2018), which may have therefore conditioned the results. Fourth, major considerations must be given to the lack of assessment of biological measures among the above-mentioned studies, since only three studies evaluated biological measures, such as endocannabinoid (Leweke et al. Reference Leweke, Piomelli, Pahlisch, Muhl, Gerth, Hoyer, Klosterkötter, Hellmich and Koethe2012) or CBD plasma levels (McGuire et al. Reference McGuire, Robson, Cubala, Vasile, Morrison, Barron, Taylor and Wright2018) and cerebral blood flow changes (Crippa et al. Reference Crippa, Nogueira Derenusson, Borduqui Ferrari, Wichert-Ana, Duran, Martin-Santos, Vinícius Simões, Bhattacharyya, Fusar-Poli, Atakan, Santos Filho, Freitas-Ferrari, McGuire, Zuardi, Busatto and Hallak2011).
Nonetheless, all together, such preliminary evidences suggest that CBD may have an effective therapeutic role in the treatment of psychiatric disorders. However, further and larger randomised placebo-controlled clinical trials studies should be performed considering not only the clinical outcomes of CBD administration, but also its effect on biological parameters such as neurotransmitter signalling, pharmacokinetics, structural and functional cerebral modifications. This approach may help to clarify the wide spectrum of action of this new molecule in psychiatric disorders.
Acknowledgements
This study was partially supported by a grant from the AIFA (Proposal 2016-02364852).



