Article contents
III.—The Magnesian Limestone of Durham
Published online by Cambridge University Press: 01 May 2009
Extract
Conditions of Deposition — Former Presence of Sulphates — Formation of “ Demagnesified ” Rocks—Development of Concretionary and Segregated Structures, etc.
Information
- Type
- Original Articles
- Information
- Copyright
- Copyright © Cambridge University Press 1919
References
page 452 note 1 In Part I of this paper a general acquaintance with the divisions of the Magnesian Limestone and with certain structural features of this rock is presupposed; readers not having such preliminary knowledge will find these subjects summarized and discussed in Part II.
page 452 note 2 Proc. Univ. Durham Phil. Soc., vol. iv, pt. v, 1911–12.Google Scholar
page 453 note 1 Q.J.G.S., vol. lxix, pp. 184–218, 1913, and vol. lxx, pp. 232–65, 1914.Google Scholar
page 453 note 2 “Borings at Cotefield Close and Sheraton”: Geol. Mag., 03, 1919, pp. 163–70.Google Scholar
page 454 note 1 Trechmann, , Q.J.G.S., vol. lxx, p. 250.Google Scholar
page 454 note 2 “On Oolites and Spherulites”: Bucher, H., Journ. of Geol., vol. xxvi, No. 7, 10–11, 1918.Google Scholar
page 454 note 3 Current bedding is traceable locally in the Roker oolite, and I have noticed it slightly developed in an altered oolite in Silkworth Quarry.
page 455 note 1 Analysis by Browell, and Kirkby, , Nat. Hist. Trans., vol. i, pt. xi, p. 204, 1866.Google Scholar
page 455 note 2 Q.J.G.S., vol. lxx, p. 232, 1914.Google Scholar
page 455 note 3 Chemical and Geological Essays, 1875.
page 455 note 4 “The Permian Formation of N.E. England”: Mid. Nat., vol. v, p. 202, 1881.Google Scholar
page 455 note 5 Regarded as Trias by some geologists.
page 455 note 6 Anhydrite and gypsum have been proved by borings in the Permian at Seaton Carew (Wilson, , Q.J.G.S., vol. xliv, p. 781, 1888Google Scholar); Whitehaven, near Norton (Tate, , Q.J.G.S., vol. xlviii, p. 488, 1892CrossRefGoogle Scholar); Hartlepool (Trechmann, , Q.J.G.S., vol. lxix, p. 184, 1913CrossRefGoogle Scholar); Leeming Lane; near Stockwith, North Lincolnshire (Dunston, G., Fed. Inst. Min. Eng., vol. xii, p. 578, 1896–7Google Scholar); in borings in South Yorkshire. Concealed coalfield of Yorkshire and Nottingham (Gibson, Walcot, Mem. Geol. Surv., 1913Google Scholar); at Market Weighton (Gibson, Walcot, Summary of Progress, Geol. Surv., p. 43, 1917Google Scholar). Trechmann gives the following analysis of the Seaton Carew boring (Q.J.G.S., vol. lxix, p. 193, 1913Google Scholar):—

The mass of anhydrite and gypsum at Hartlepool was 267 ft. 2 in. It was overlaid by glacial deposits, so it may have been originally much thicker.
page 456 note 1 A considerable thickness of limestone (some 200 feet) appears to be absent on the west side of Cleadon Hills, and it may be that a thick bed of sulphate has been removed here. The extensive slipping down of beds and consequent brecciation along the eastern side of the Reef, which can be seen at several places along the Durham coast, is most probably due to this cause. Trechmann has noted several vertically slickensided surfaces (Q.J.G.S., vol. lxix, p. 201Google Scholar).
page 456 note 2 Geodes containing gypsum with dolomitic rhombs disseminated through them have been observed by Trechmann.
page 456 note 3 Anhydrite was deposited in periods of drought and higher temperature. See discussion of this subject by Smith, B. in “The Chellaston Gypsum-breccia and its relation to the Gypsum-anhydrite deposits of Britain” : Q.J.G.S., vol. lxxiv, p. 195.Google Scholar
page 457 note 1 Trechmann gives an analysis of the Great Marl bed of Fulwell Quarries—

page 457 note 2 Trechmann gives an analysis of dolomitic oolite from Haswell—
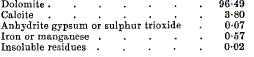
page 457 note 3 The coarse granular dolomite occurring in the Cotefield Close boring gives (analysis by A. D. N. Bain, B.Sc.)—

page 457 note 4 [For note see next page.]
page 457 note 5 Silicified limestones occur at the south end of Marsden Bay, Hendon, etc., and Trechmann has noticed silicified oolitic limestones.
page 458 note 4 Parts of the Bryozoa Reef have been dolomitized, e.g. Trechmann records the shell substance of a Productus as containing 95.88 per cent of dolomite, and of an Arca 98.68. Much of the sediment which fell on the reef was calcareous, and there is little doubt that the highly dolomitic nature of parts of the reef is due to subsequent dolomitization. This may have gone on penecontemporaneously with deposition by the magnesium waters of the sea, lime being removed and replaced by magnesia, in the same way that the coral reefs of the present day have been proved to undergo a process of dolomitization (Judd, “Atoll of Funafuti,” Royal Soc. London, 1904). Certain observations on the decalcified rocks in the reef, however, lead me to think that the dolomitization of parts of it may have taken place long after deposition. It is possible, if the solution changes which are discussed in this paper have taken place, that the magnesium may have been transferred from one part of the limestone to another. This appears to be so in the case of parts of the reef, and in some of the dolomitized highly calcareous rocks known as “bluestones” both in the upper and lower limestones. I have lately examined breccias occurring in the lower calcareous limestones of Raisby Hill Quarry which have been formed by the alteration of these rocks by magnesium and other solutions (see Breccias, Part II of this paper).
page 458 note 1 Portions of the reef have been partially decalcified by manganese solutions, Tunstall Hill, Fox Cover Quarry. Beacon Hill. Trechmann gives an analysis of a decalcified rock from Tunstall Hill—
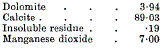
page 459 note 1 Q.J.G.S., vol. lxx, p. 264.Google Scholar
page 459 note 2 Ibid., p. 253.
page 459 note 3 This section is noticed in my paper on “The Permian of N. Durham”, op. jam. cit., p. 267, 1912Google Scholar, and is drawn and described in fig. 10, where I refer to the change as having been brought about by the leaching out of the magnesium carbonate from the dolomite. Dr. Trechmann also fully describes it (Q.J.G.S., vol. lxx, p. 252, 1914Google Scholar), and explains the alteration as being due to the solution of dolomite. The amount of interstitial calcite in the Flexible Limestone is usually very small, so that solution of dolomite demands the solution of almost the entire rock and its replacement by calcite, e.g. Flexible Limestone, Marsden, interstitial calcite 0·71. The analyses given by Dr. Trechmann of this bed above the thrust plane at Hendon are—

page 460 note 1 This section is drawn and described in my paper on “The Permian of N. Durham”, op. jam cit., fig. 10.
page 462 note 1 The concretionary limestones often contain organic matter and are sometimes strongly fetid.
page 462 note 2 Stocks, Q.J.G.S., vol. lviii, pp. 46–58, 1902.Google Scholar
page 462 note 3 Univ. Durham Phil. Soc. Proc., vol. iv, pt. v, p. 271, 1911–12.Google Scholar
page 462 note 4 Q.J.G.S., vol. lxix, p. 198, 1913.Google Scholar
page 463 note 1 Trechmann lays emphasis on the very fine state of the powdery material in both the concretionary and segregated rocks (Q.J.G.S., vol. lxx, pp. 252, 256).Google Scholar
page 464 note 1 Trechmann gives the following analyses from the coast near Horden—
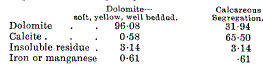
page 464 note 2 An analysis of a hand specimen by the late R. C. Burton, B.Sc., of fossiliferous dolomite passing into a segregated limestone from which the fossils were obliterated gave—
In both the unaltered rocks the interstitial calcite is small, in the first case exceedingly so.
page 465 note 1. Where statements are made regarding the composition of rocks and analyses were not available they have always been analysed, generally by postgraduate students of Armstrong College.
page 465 note 2. In connexion with solution changes in the magnesium limestone, it is perhaps worthy of note that Sunderland Water contains on the average—

As a very large and busy manufacturing district obtains all its water from this formation, large quantities of both calcium and magnesium must yearly be dissolved out of the magnesian limestone.
- 12
- Cited by

