1. Introduction
The Iberian Pyrite Belt (IPB), located at the southwest margin of the Iberian Peninsula, contains one of the largest accumulations of massive sulphide deposits in the world. The original reserves of this Devonian-Carboniferous basin have been estimated at 1700 Mt of massive sulphides and 300 Mt of stockwork-type mineralization. Ores are primarily composed of pyrite with minor amounts of base metal sulphides (chalcopyrite, sphalerite and galena), other sulphides (including arsenopyrite) and sulfosalts. Besides Cu, Zn and Pb, important reserves of Sn, Au and Ag have been also documented. Mining exploitation in the region dates back over 5000 years. During this time, 82 sulphide deposits have been mined and several other indications have been documented (Pinedo Vara Reference Pinedo Vara1963; Sáez et al. Reference Sáez, Almodóvar and Pascual1996; Reference Sáez, Almodóvar and Barriga1997; Reference Sáez, González, Donaire, Toscano, Yesares, Almodóvar and Moreno2024; Leistel et al. Reference Leistel, Marcoux, Thiéblemont, Quesada, Sánchez, Almodóvar, Pascual and Sáez1998; Tornos et al. Reference Tornos, Barriga, Marcoux, Pascual, Pons, Relvas, Velasco, Large and Blundell2000; Almodóvar et al. Reference Almodóvar, Yesares, Sáez, Toscano, González and Pons2019; Sáez et al., Reference Sáez, González, Donaire, Toscano, Yesares, Almodóvar and Moreno2024).
At the latest Devonian, the environmental conditions of the hitherto stable IPB were drastically conditioned by the basin breakup and compartmentation associated with the onset of the Variscan cycle in the region (Moreno et al., Reference Moreno, Sierra, Sáez, Strogen, Somerville and Jones1996; Soriano & Casas, Reference Soriano and Casas2002; Oliveira et al. Reference Oliveira, Pereira, Carvalho, Pacheco and Korn2004, Reference Oliveira, Quesada, Pereira, Matos, Solá, Rosa, Albardeiro, Díez-Montes, Morais, Inverno, Rosa, Relvas, Quesada and Oliveira2019). The resulting IPB configuration consisted of a puzzling framework of coeval sub-basins filled with black shales, variable amounts of volcanic rocks and, on many occasions, large accumulations of massive sulphides (Moreno et al. Reference Moreno, Sierra, Sáez, Strogen, Somerville and Jones1996; Leistel et al. 1998; Sáez et al. Reference Sáez, Pascual, Toscano and Almodóvar1999). These sub-basins, currently obscured by tectonic overprint, achieved disparate environmental conditions, and their black shales generally contain abundant spores and organic-walled microphytoplankton (González et al. Reference González, Playford and Moreno2005a ; Reference González, Moreno and Playford2005 b; Sáez et al. Reference Sáez, Moreno and González2008; Pereira et al. 2021; Reference Pereira, Matos, Mendes, Solá, Albardeiro, Morais, Araújo, Pacheco and Oliveira2023; Mendes et al. Reference Mendes, Pereira, Matos, Albardeiro, Morais and Araújo2024). Among the marine organisms, the most prominently reported species is Maranhites mosesii (Sommer) Brito 1967 emend Gonzalez 2009.
Other microphytoplankton species found in the IPB, including acritarchs and prasinophyte microalgae, can be locally abundant, but for yet unknown reasons M. mosesii is by far the most widely and abundantly reported species in the region (González et al. Reference González, Playford and Moreno2005; González, Reference González2009; Mendes et al. Reference Mendes, Pereira, Matos, Albardeiro, Morais and Araújo2024).
According to González (Reference González2009), this microphytoplankton species exhibits high morphological variability linked to its ontogenetic development. However, while intra-sample size variations may be attributed to this growth trend, M. mosesii specimens from the IPB show significant inter-sample size disparities that are difficult to explain exclusively by this phenomenon. The present study provides the biometric analysis of populations of M. mosesii obtained from different localities, specifically from coeval massive sulphide-generating environments (MSGE) and non-massive sulphide-generating environments (non-MSGE). Geochemical analysis of the hosting black shales and Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) spectra of a selection of specimens representative of each environment provide insights into the origin of this morphological disparity. The casual/causal relationship between morphological variation and presence/absence of massive sulphides in the hosting environments is thus explored.
To date, the information provided by the palynological analysis of the black shales hosting the IPB massive sulphides has been instrumental in biostratigraphically constraining the oldest mineralization event. Miospore assemblages invariably attributed to the uppermost Devonian Biozone of Western Europe, Retispora lepidophyta-Verucosisporites nitidus (LN) by Streel et al. (Reference Streel, Higgs, Loboziak, Riegel and Steemans1987), have been reported in the shales hosting the massive sulphides of Aznalcóllar (Pereira et al. Reference Pereira, Sáez, Pons, Oliveira and Moreno1996), Neves-Corvo (Pereira & Pacheco, Reference Pereira and Pacheco2004, Reference Pereira, Matos, Solá, Batista, Salgueiro, Rosa, Albardeiro, Mendes, Morais, de Oliveira, Pacheco, Araújo, Castelo Branco, Neto, Lains Amaral, Inverno and Oliveira2021; Mendes et al. Reference Mendes, Pereira, Matos, Albardeiro, Morais and Araújo2024), Tharsis (González et al. Reference González, Moreno and Sáez2002), Sotiel Coronada (González et al. González et al., Reference González, Moreno and Santos2006), Torerera, Las Herrerías (Sáez et al., Reference Sáez, Moreno and González2008), Caveira (Matos et al. Reference Matos, Pereira, Rosa and Oliveira2014), Semblana-Rosa Magra and Monte Branco (Pereira et al. Reference Pereira, Matos, Solá, Batista, Salgueiro, Rosa, Albardeiro, Mendes, Morais, de Oliveira, Pacheco, Araújo, Castelo Branco, Neto, Lains Amaral, Inverno and Oliveira2021). The multi-analytical approach here proposed represents a step forward, as it examines the viability of considering the morphology of M. mosesii as a prospecting method to discriminate between mineralized and barren sequences within the IPB.
2. Geological setting
The IPB is the largest and most representative domain comprising the South Portuguese Zone in the southern margin of the Iberian Variscan Massif (Figure 1a). Its stratigraphic record extends from Middle Devonian to Late Mississippian (González et al. Reference González, Moreno, López, Dino and Antonioli2003; Mendes et al. Reference Mendes, Pereira, Matos, Albardeiro, Morais, Solá, Salgueiro, Pacheco, Araújo, Inverno and Oliveira2020; Reference Mendes, Pereira, Matos, Albardeiro, Morais and Araújo2024; Moreno & González, Reference Moreno, González and Vera2004; Pereira et al. Reference Pereira, Matos, Solá, Batista, Salgueiro, Rosa, Albardeiro, Mendes, Morais, de Oliveira, Pacheco, Araújo, Castelo Branco, Neto, Lains Amaral, Inverno and Oliveira2021) comprising three lithostratigraphic units (Schermerhörn, Reference Schermerhörn1971). From footwall to hanging wall, these are: Phyllite-Quartzite Group (PQ Group), Volcanic-Sedimentary Complex (VSC) and Culm Group (referred to in Portugal as Baixo Alentejo Flysch Group) (Figures 1a and 2). The PQ Group consists of a thick detrital sequence of shales and sandstones, Givetian–latemost Famennian in age, deposited in a stable and shallow siliciclastic platform, eventually affected by storms (Sáez & Moreno, Reference Sáez and Moreno1997; Matos et al. Reference Matos, Pereira, Rosa and Oliveira2014; Oliveira et al. Reference Oliveira, Rosa, Rosa, Pereira, Matos, Inverno and Andersen2013; Reference Oliveira, Quesada, Pereira, Matos, Solá, Rosa, Albardeiro, Díez-Montes, Morais, Inverno, Rosa, Relvas, Quesada and Oliveira2019). To the top, the series incorporates isolated limestone bodies (see, for example, van den Boogaard, Reference Boogaard1963; van den Boogaard & Schermerhörn, Reference Boogaard and Schermerhörn1981; Albardeiro et al. Reference Albardeiro, Matos, Mendes, Solá, Pereira, Morais, Salgueiro, Pacheco, Araújo and Oliveira2020; Mendes et al. Reference Mendes, Pereira, Matos, Albardeiro, Morais, Solá, Salgueiro, Pacheco, Araújo, Inverno and Oliveira2020) and conglomerates, as well as mega debris-flow and fan-delta deposits, all in relation with the instability affecting the IPB during early propagation of the Variscan cycle (Moreno et al. Reference Moreno, Sierra, Sáez, Strogen, Somerville and Jones1996). Such instability resulted in the Late Devonian fragmentation and compartmentation of the IPB basin. The transition between the PQ Group and the VSC is commonly associated with a black shale sequence rich in organic matter including minor interbedded volcanics. This so-called anoxic sequence (Moreno & González, Reference Moreno, González and Vera2004) hosts many of the large massive sulphide deposits from the southern part of the region, including Tharsis, Sotiel Coronada-Migollas, Aznalcóllar-Los Frailes, Neves-Corvo, Lousal and Carveira. Above the anoxic sequence, the VSC incorporates a diversity of Mississippian sedimentary volcanic and subvolcanic rocks, mafic and felsic in nature, displaying frequent thickness variations and abrupt lateral and vertical facies changes. This unit is interpreted in relation to the transtensional effect of the early syn-orogenic stage occurring in a heterogeneous and compartmentalized basin (Moreno et al. Reference Moreno, Sierra, Sáez, Strogen, Somerville and Jones1996; Martín-Izard et al. Reference Martín-Izard, Arias, Arias, Gumiel, Sanderson, Castañón and Sánchez2017; Oliveira et al. Reference Oliveira, Quesada, Pereira, Matos, Solá, Rosa, Albardeiro, Díez-Montes, Morais, Inverno, Rosa, Relvas, Quesada and Oliveira2019).
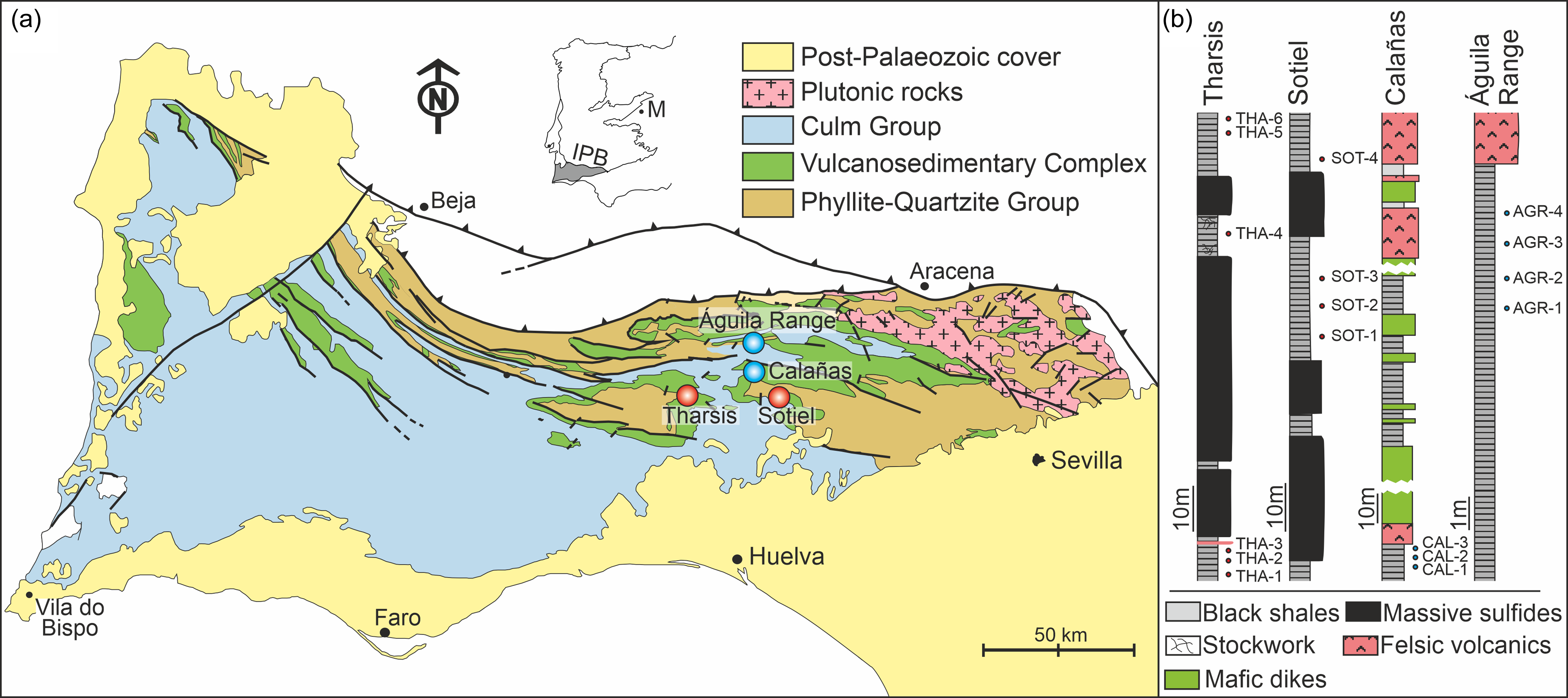
Figure 1. (a) Geological sketch map of the Iberian Pyrite Belt (IPB) with indication of the four sub-environments/sub-basins considered in this study. M, Madrid; IPB, Iberian Pyrite Belt. Red circles indicate MSGE. Blue circles indicate non-MSGE. (b) Stratigraphic logs at these localities showing the location of the studied samples. All the samples are latest Famennian (LN Biozone) in age.
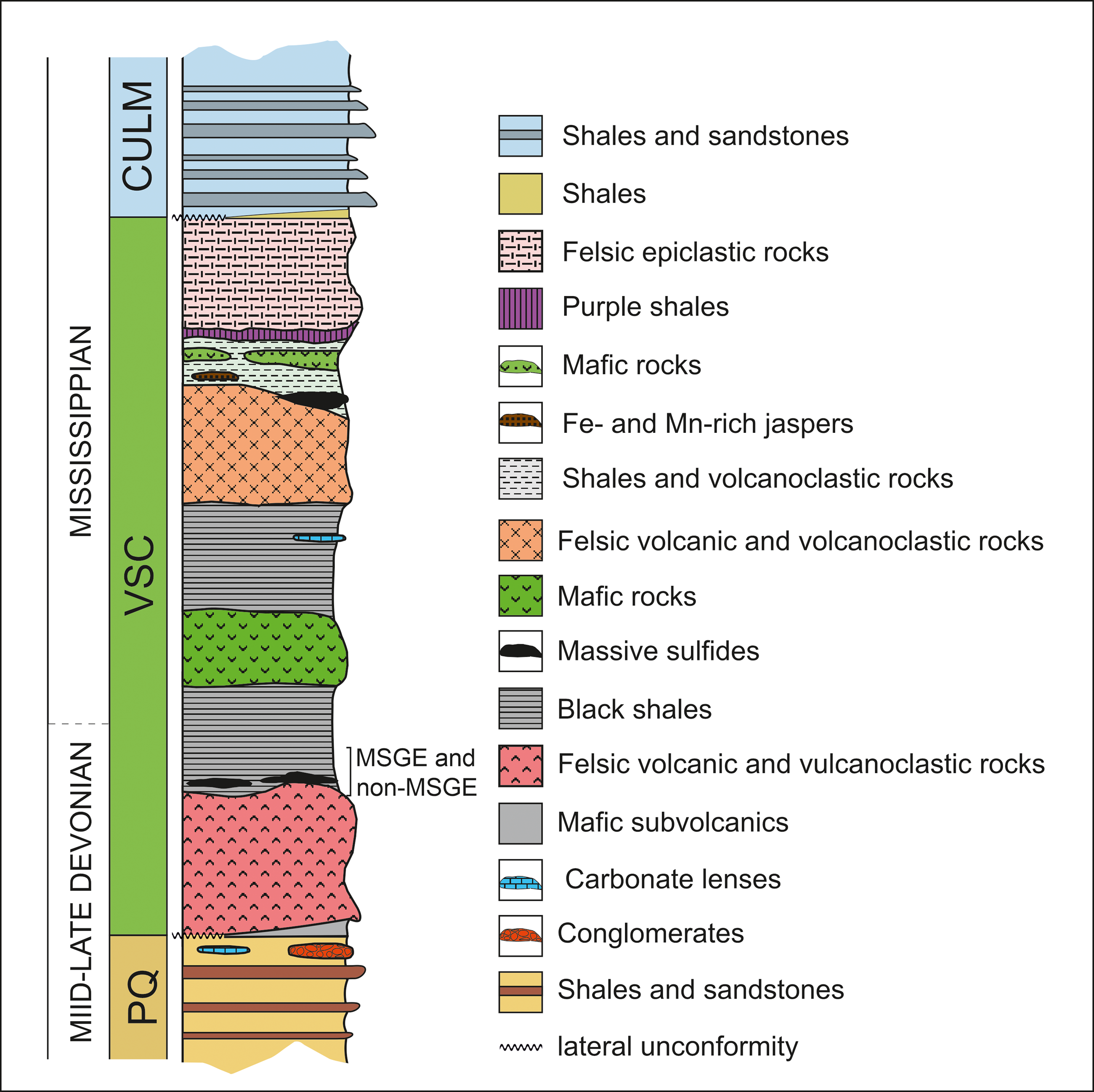
Figure 2. Synthetic stratigraphic log of the IPB.
Sulphide deposits from the north and intermediate zones of the IPB are mostly associated with volcanic and volcaniclastic rocks of the VSC. The geochronological constrain of these deposits is still poor, but limited available data indicate a late Tournaisian age (Barrie et al. Reference Barrie, Amelin and Pascual2002; Dunning et al. Reference Dunning, Díez Montes, Matas, Martín Parra, Almarza and Donaire2002; Albardeiro et al. Reference Albardeiro, Morais, Matos, Solá, Salgueiro, Pereira, Mendes, Batista, De Oliveira, Díez-Montes, Inverno, Pacheco and Araújo2023). Considering all the biostratigraphic and geochronological age data, the existence of two mineralizing pulses (late Famennian and late Tournaisian) or a single continuous metallogenic episode extending from late Famennian to late Tournaisian is still under debate (Albardeiro et al. Reference Albardeiro, Matos, Mendes, Solá, Pereira, Morais, Salgueiro, Pacheco, Araújo and Oliveira2020; 2023; Sáez et al. Reference Sáez, González, Donaire, Toscano, Yesares, Almodóvar and Moreno2024).
The contact between the VSC and the overlying Culm Group is marked by another shaly sequence, the Basal Shaly Formation (Moreno & Sequeiros, Reference Moreno and Sequeiros1989), referred to in Portugal as the Brancanes Formation (Oliveira et al. Reference Oliveira, Rosa, Rosa, Pereira, Matos, Inverno and Andersen2013, Reference Oliveira, Quesada, Pereira, Matos, Solá, Rosa, Albardeiro, Díez-Montes, Morais, Inverno, Rosa, Relvas, Quesada and Oliveira2019). This unit comprises reworked material from the uppermost VSC intervals, along with siliciclastic deposits linked to the initial post-volcanic turbiditic pulses in the region. Above this formation, the Culm Group comprises the syn-orogenic infill of the IPB basin (Moreno, Reference Moreno1993; Moreno et al. Reference Moreno, Sierra, Sáez, Strogen, Somerville and Jones1996; Oliveira, Reference Oliveira, Dallmeyer and Martínez García1990; Reference Oliveira, Quesada, Pereira, Matos, Solá, Rosa, Albardeiro, Díez-Montes, Morais, Inverno, Rosa, Relvas, Quesada and Oliveira2019). During the Asturian phase of the Variscan orogeny, the entire succession was deformed following a thin-skinned tectonic style (Silva et al. Reference Silva, Oliveira, Ribeiro, Dallmeyer and Martínez García1990).
The four sampling sites selected in this study are stratigraphically located within the anoxic sequence transitional between the PQ Group and VSC (Figure 2). All samples collected in these sites were palynostratigraphically analyzed and attributed to the uppermost Famennian LN Miospore Biozone (Streel et al. Reference Streel, Higgs, Loboziak, Riegel and Steemans1987) of Western Europe. Two of the studied sites, Tharsis and Sotiel, are located in MSGE, whereas the other two, Águila Range and Calañas, are from non-MSGE (Figure 1). Samples from non-MSGE are outcrop samples. Those from Tharsis and Sotiel are, respectively, from an open pit and from an underground mine. The stratigraphic location of the samples studied in each locality is illustrated in Figure 1b. Details of the biostratigraphic analysis carried out on each site can be seen in González et al. (Reference González, Moreno and Sáez2002, Reference González, Playford and Moreno2005, González et al., Reference González, Moreno and Santos2006) and Moreno et al. (Reference Moreno, González and Sáez2003).
3. Materials and methods
The analysis performed on all the samples includes biometry, Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS) of selected M. mosesii specimens and geochemical analysis of the hosting black shales. Biometry and TOF-SIMS were preceded by a palynological extraction procedure, which essentially followed the standard tri-acid digestion method described by Wood et al. (Reference Wood, Gabriel, Lawson, Jansonius and McGregor1996). This comprises initial disaggregation of approx. 35 g of sample, immersion in HCl (36%) to remove carbonates, dissolution of silicate minerals with hot HF (40%) and a second immersion into hot HCl (36%) to eliminate fluorides and remaining carbonates. After neutralization, the organic residue was oxidized with Schulze solution for variable time intervals. The oxidized residue was mounted in microscope slides using Cellosize as dispersant and Elvacite as mounting medium. Preservation status of the selected samples was optimal for palynological identification. After biostratigraphic recognition and palynofacies analysis, based on 350 counts, the biometry of M. mosesii was performed selecting 120 specimens per sample, when possible. The morphological features measured were: vesicle diameter, equatorial pads, equatorial embayment, lateral thickenings and translucent bladders. Details on these structural elements can be seen in Figure 3 and in González (Reference González2009). Biostratigraphy, palynofacies and biometric analyses were performed in the Department of Earth Sciences, University of Huelva, using a Nikon Labaphot-2 microscope equipped with the digital camera Nikon DS-Fi1 (Software NIS Elements, F Package, 3.22.00). Processed and remaining unprocessed samples are deposited in the collections of the Earth Sciences Department, University of Huelva.
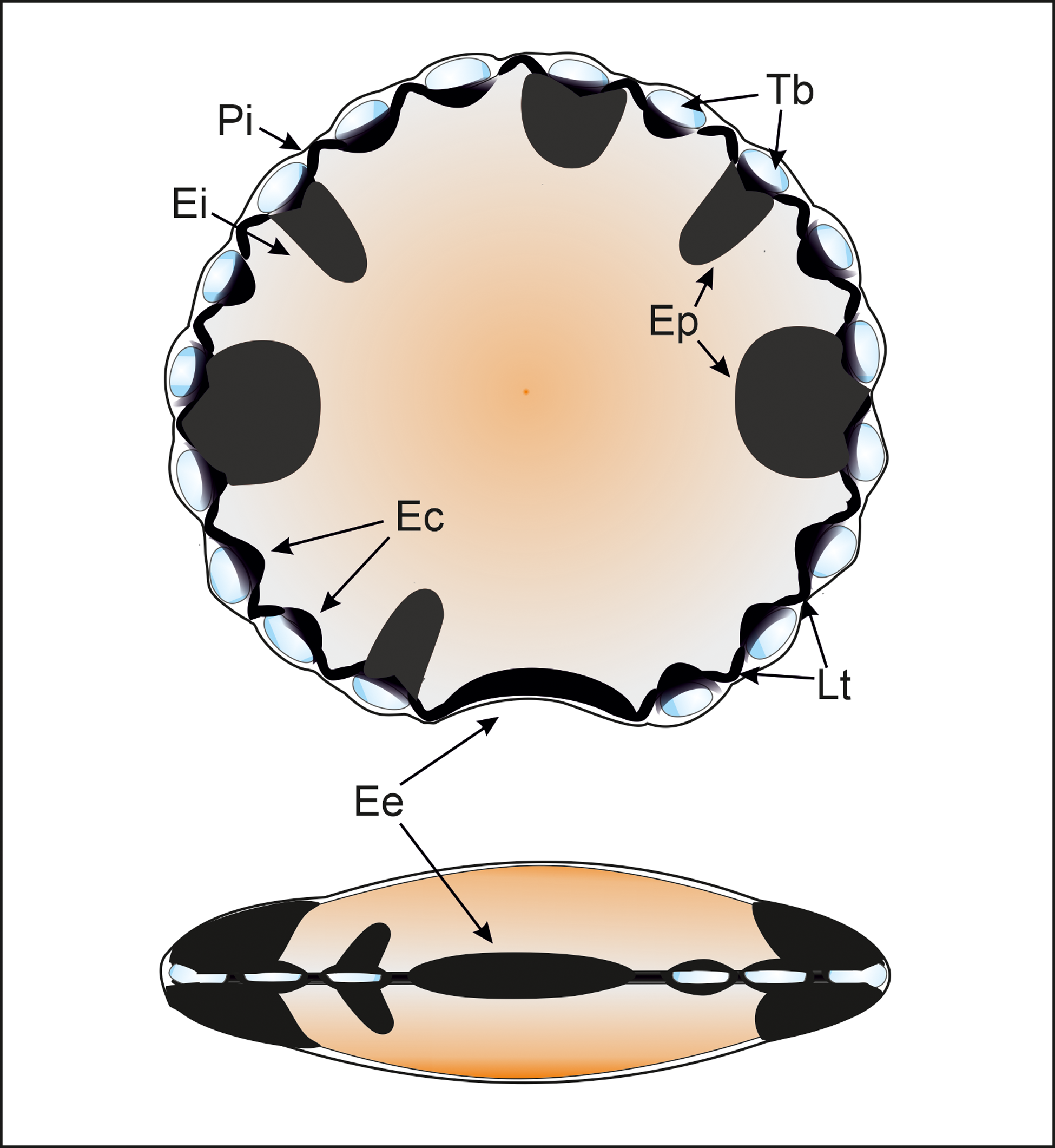
Figure 3. Polar and equatorial scheme views of M. mosesii after González (Reference González2009) with indication of its main equatorial structures: (Pi) Perieilyma. Outer vesicle wall; (Ei) Endeilyma. Inner vesicle wall; (Ep) Equatorial pads. Solid, protrusive discrete to rarely interconnected structures, circular to subcircular in outline; (Ec) Embracing cells. Dark equatorial structures, elliptical to subircular in outline, that enclose translucent bladders and are connected by lateral thickening; (Lt) Lateral thickening. Small, solid, circular, subcircular or arcuate structures that connect embracing cells; (Tb) Translucent bladders. Hollow, rounded to elliptical equatorial structures, enclosed by embracing cells, that progressively enlarge during vesicle maturation; (Ee) Equatorial embayment, normally thickened, that may or may not be accompanied by embracing cells or equatorial pads.
For the TOF-SIMS analysis, 20–40 specimens per sample of M. mosesii were picked with a micromanipulator needle and mounted in a double-sided Cu tape. Measurement and chemical imagery were then performed using a TOF-SIMS IV instrument (ION-TOF GmbH, Germany) located at the Scientific and Technological Research Assistance Centre of the University of Vigo, Spain. The analysis was carried out with a primary ion beam of 25 KeV Bi3+ (pulsed current between 0.12 and 0.15 pA) scanning over a 500 × 500 μm2 area for 100–150 s. The Primary Ion Dose Density was settled to be below the static limit (1013 ions/cm2). A pulsed electron flood gun source was employed for surface build up charge compensation.
Spectra (m/z 0–1000) and chemical maps of selected m/z values (scans acquisition repeated 75 times) were collected for all samples in positive and negative polarity. Positive and negative ion spectra were internally mass-calibrated using CH₃+, C₂H₃+, C₃H₅+ and C₅H₁₄NO⁺ peaks for positive ions, and CH−, C₂H−, C₄H− and C₁₈H₃₅O₂− peaks for negative ions, respectively. Deviation between theoretical and measured masses was always below 100 ppm. Combining the elements described in the literature as potential contaminant for phytoplankton with those that could be mobilized in oxygen-depleted and/or hydrothermally influenced environments, the list of major and trace elements searched in TOF-SIMS profiles includes Mg, Al, Si, Cr, Fe, Co, Zn, As, Cd, Cs, Ba, Ce, Cu, Hg and Pb.
Chemical composition of shales was obtained after milling on agate mortar and split into 20 g fractions at the University of Huelva laboratories. Geochemistry was characterized by analyzing major and trace elements together with carbon and sulphur in a fully certified commercial laboratory (ACME, Analytical Laboratories, Vancouver, Canada). Total abundances of major oxides were determined by ICP emission spectrometry. Rare Earth and refractory elements were analyzed by ICP mass spectrometry. Both procedures followed Li-metaborate/tetraborate fusion and dilute nitric digestion of samples weighing 0.2 g. Sample splits of 0.5 g were analyzed for a selection of metals and semi-metals including Cu, Pb, Zn, As, Se and Hg after aqua regia digestion. Sulphur, total C and organic C were determined by Leco analysis.
4. Results
4.a. Maranhites mosesii
The microphytoplankton species M. mosesii extends from Early Devonian to Mississippian. It is widely distributed throughout Gondwana and southern margin of Laurussia and is generally associated with shallow marine depositional environments, relatively close to emergent landmasses (González, Reference González2009). Morphologically, it consists of a vesicle, originally discoidal, circular to subcircular in outline, 40 to 200 μm in diameter and two-layered. The inner layer (endeilyma), noticeably thicker than the outer layer (perieilyma), contains well-differentiated structural elements at equatorial positions, including solid pads, embracing cells, transparent bladders, lateral thickenings and an equatorial embayment (Figure 3).
The ontogenetic trend detected in M. mosesii (González, Reference González2009) entails increasing vesicle size and continuous transformation of these equatorial structures. Growth trends also involving vesicle enlargement have been described in cyst-forming prasinophyte species during their non-motile stage (Tappan, Reference Tappan1980; Knoll et al. Reference Knoll, Swett and Mark1991; Teyssèdre, Reference Teyssèdre2006; Taylor et al. Reference Taylor, Taylor and Krings2009). As in prasinophytes, the progressive cyst enlargement of M. mosesii cannot be solely attributed to the consumption of internal reserves, necessitating metabolic exchange with the surrounding environment.
The biometric analysis of M. mosesii shows that the average equatorial diameter and the number of pads-bearing specimens clearly differ between MSGE and non-MSGE (Figure 4). In samples from the two MSGE selected in this study (Figures 1 and 2), the average equatorial diameter of M. mosesii specimens ranges from 68.2 to 73.6 μm and 70.7 to 76.7 μm, respectively, whereas in non-MSGE, this increases up to 81.7 to 92 μm and 86 to 102 μm. The results show that the specimens in MSGE are about 15% smaller than in non-MSGE (Figures 4 and 5). Considering the number of pad-bearing specimens, the percentage observed in MSGE ranges from 3.0% to 32.9%, whereas in non-MSGE, it varies between 43.2% and 52.8%. This indicates a reduced occurrence of pad-bearing specimens in MSGE compared to non-MSGE. There is only one sample from MSGE that, with 47.1% of pads-bearing specimens and 88.1 μm of average equatorial diameter, clusters better with those from non-MSGE. The rest of structural elements measured in M. mosesii evidence variations among samples with no clear tendencies or signs of clustering (Supplementary Table 1).
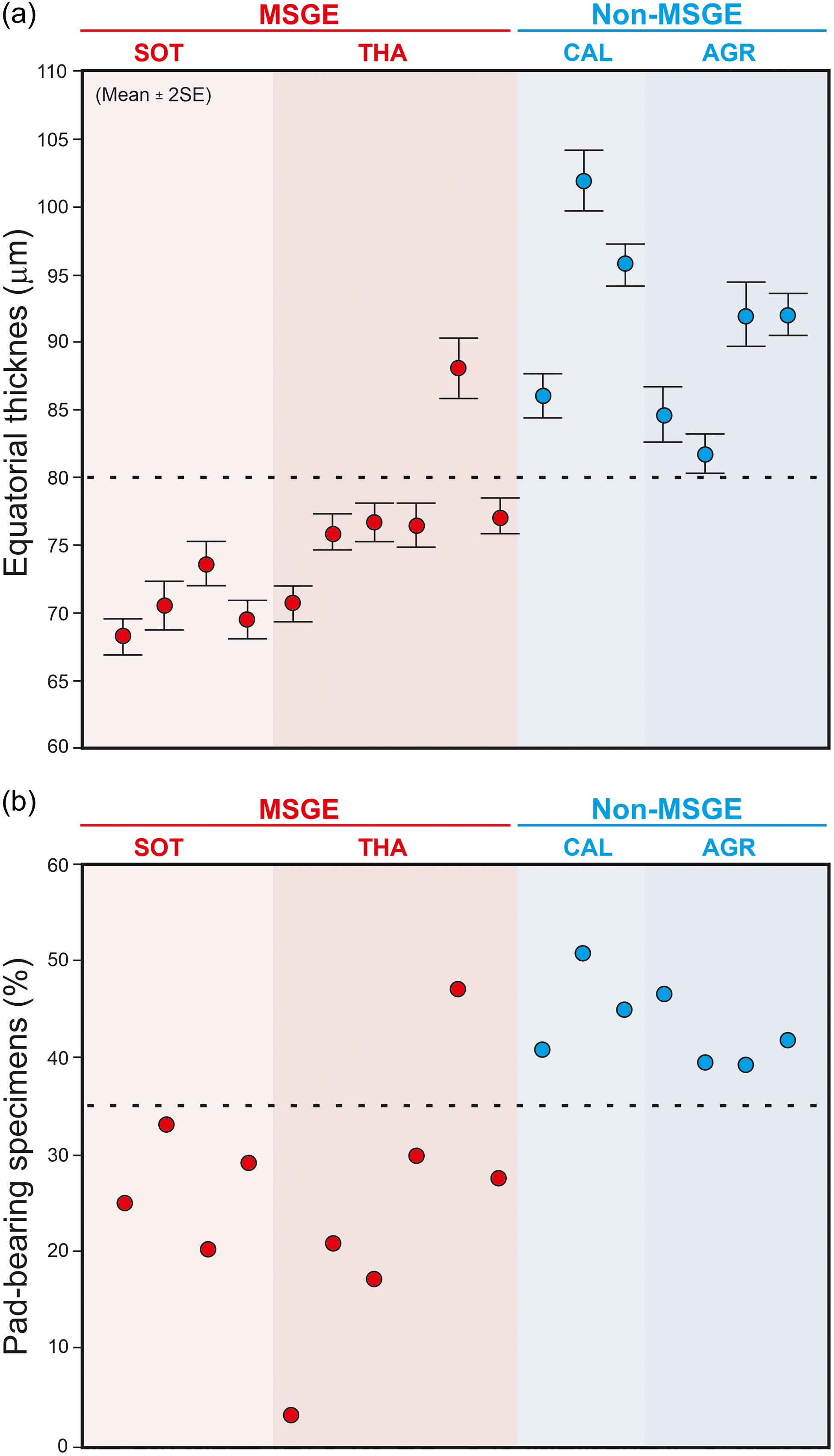
Figure 4. Average equatorial size (a) and percentage of pad-bearing specimens of M. mosesii (b) in samples from MSGE and non-MSGE. SOT, Sotiel; THA, Tharsis; CAL, Calañas; AGR, Águila Range.
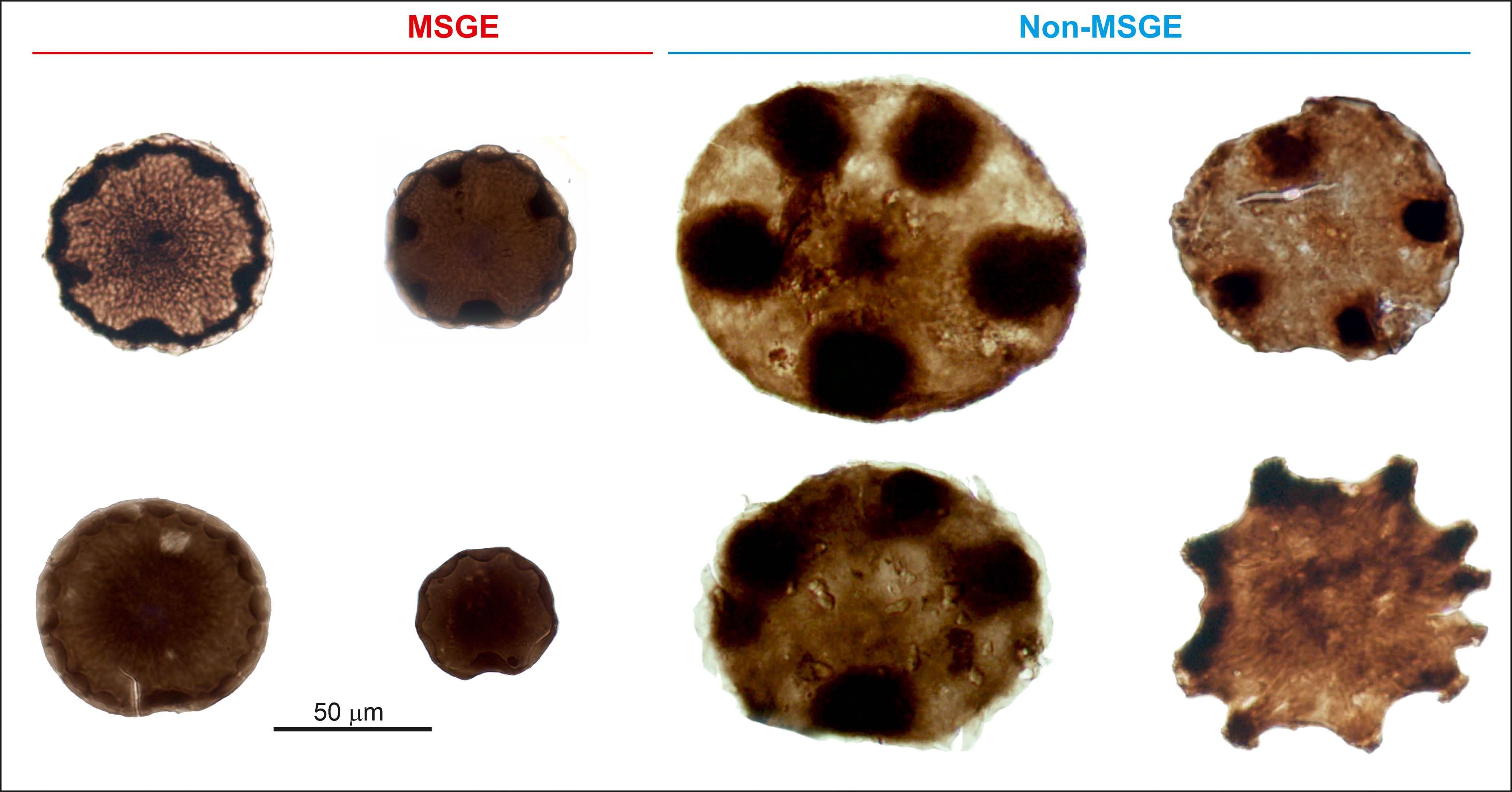
Figure 5. Specimens of M. mosesii recovered in MSGE and non-MSGE showing clear differences in equatorial size.
4.b. Inorganic element fixation during cyst stage
The elements assimilated by M. mosesii detected with TOF-SIMS spectra are shown in Figure 6. The most striking feature concerns the behaviour of As. This element is commonly detected, with variable intensity, in all but three MSGE samples, but it is absent or shows very low concentration in most of the samples from non-MSGE. Other elements, including Mg, Al, Si, Fe, Zn, Co, Cs, Cr, Ba and Pb, were detected virtually in all TOF-SIMS spectra, but with variable intensities and no differentiation between MSGE and non-MSGE. Of these, Si shows intense peaks in all cases, Co is the element with the highest variability and Ba and Ce depict very similar behaviour, drawing a distribution pattern apparently not related to the environmental differentiation followed in this study. The rest of the elements analyzed, i.e., Cu, Hg, Ce and Cd, were absent, detected randomly or with very low intensity. The elemental chemical TOF-SIMS images (Figure 7) show that the level of enrichment in As, Fe and Al occasionally differs among specimens from a given sample, indicating that these elements were gradually assimilated by M. mosesii during cyst growth. Such contrasting occurrences exclude the influence of diagenetically controlled enrichment mechanisms. Other elements, including Si, Mg, Co, Cs and Pb, remain fairly constant in all the specimens of a given sample, suggesting that the enrichment mechanism was in these cases related to a generalized post-depositional process. At the level of specimen, the TOF-SIMS shows no compositional variations across the surface, indicating that the outer wall of M. mosesii has a uniform chemical structure and that the elemental enrichment, either at cyst stage or during post-depositional assimilation, was also uniform.
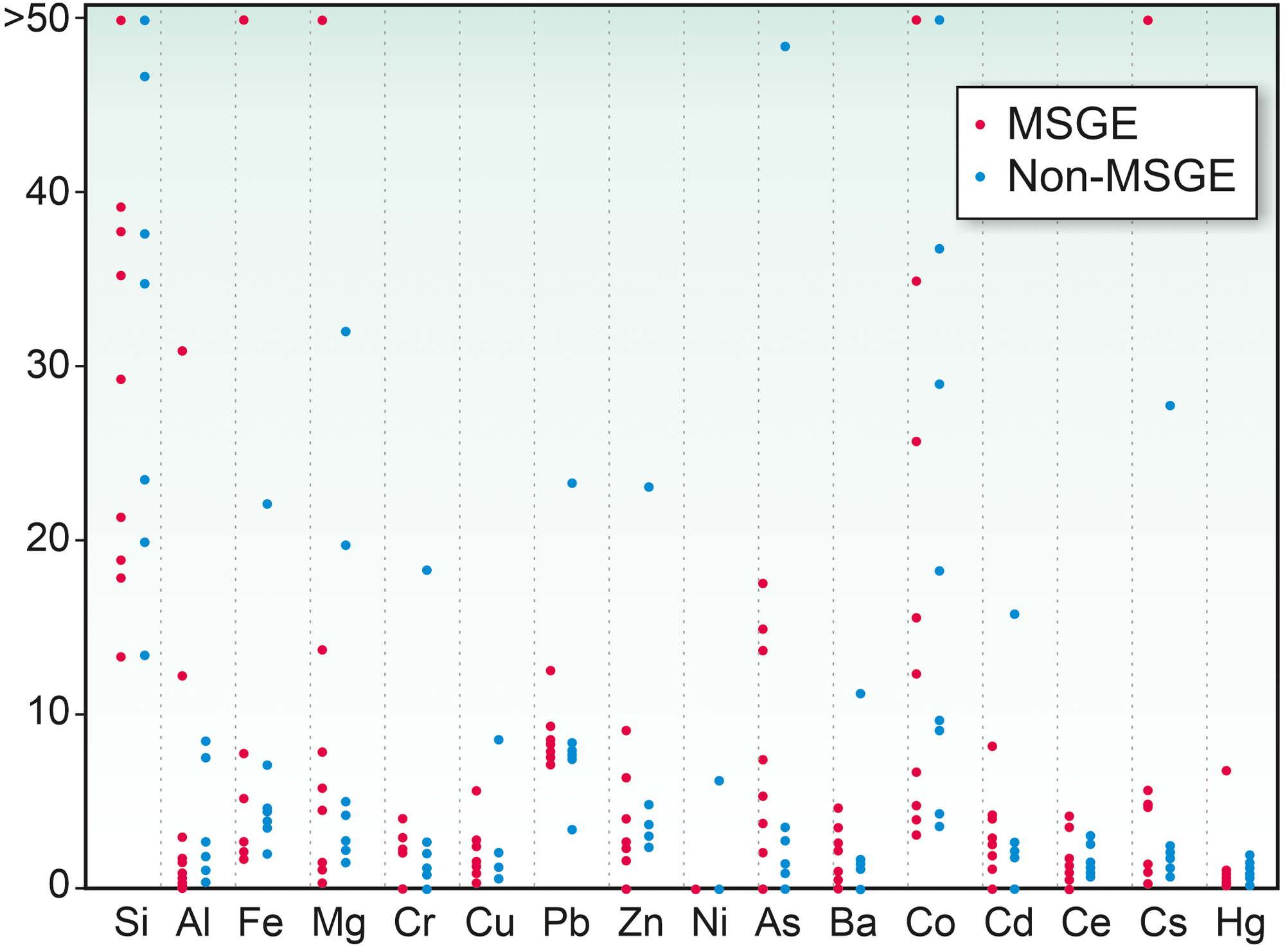
Figure 6. Elements detected in M. mosesii from MSGE and non-MSGE based on TOF-SIMS spectra. Vertical scale represents enrichment factor with respect to the holder (adhesive tape). Spectra normalized to PDMS peaks (m/z 147 and 207) identified in the holder tape.
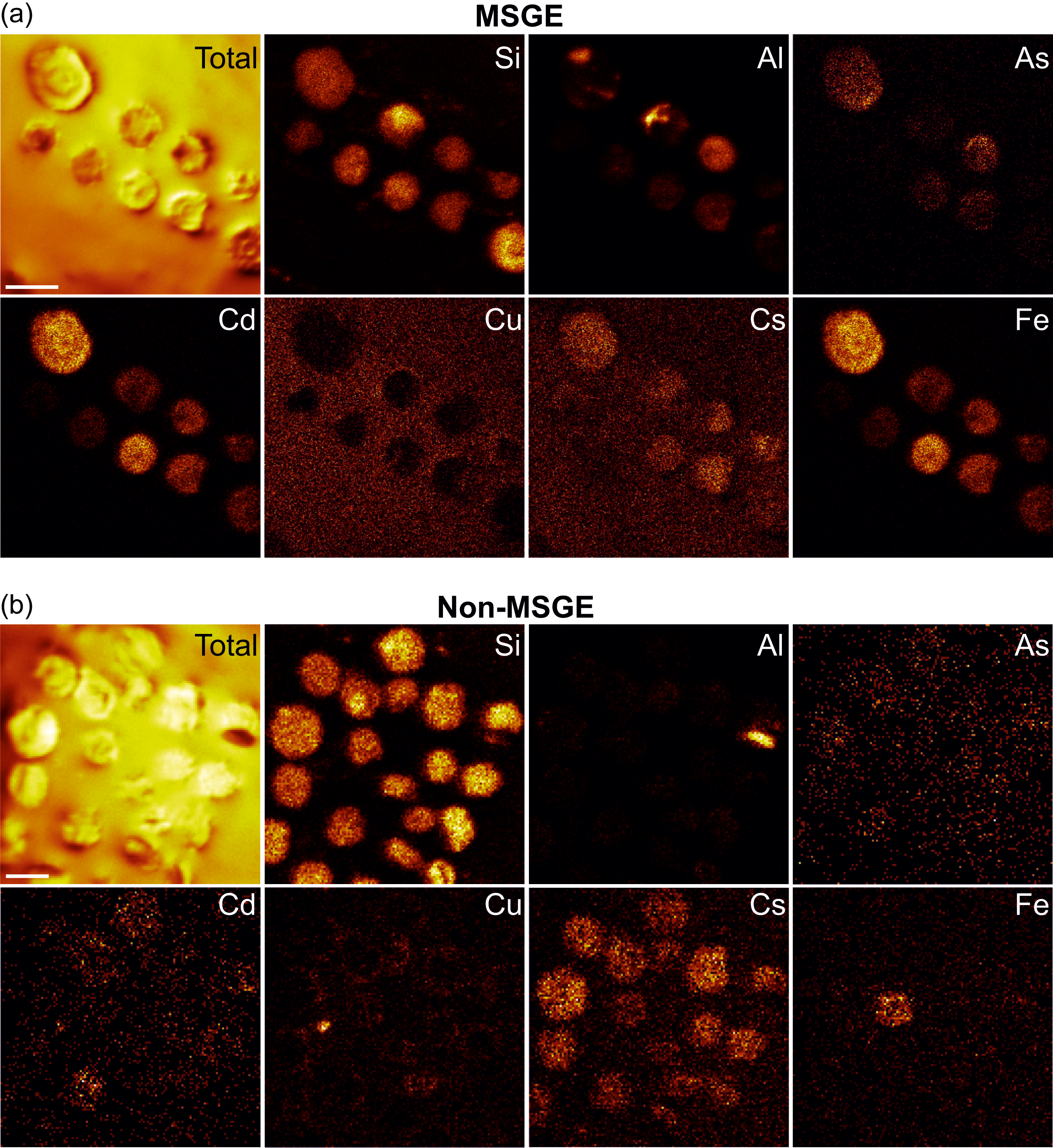
Figure 7. TOF-SIMS chemical images of selected elements detected in specimens of M. mosesii from sample THA-4, as representative of MSGE (a) and sample AGR_1, as representative of non-MSGE (b). Scale bars 100 μm.
4.3. Seafloor environment reconstruction
Geochemical analysis of the black shales hosting the cysts of M. mosesii indicates oxygen-deficient conditions according to Hatch and Leventhal Reference Hatch and Leventhal(1992) and Hoffman et al. (Reference Hoffman, Akgeo, Maynard, Joachimski, Hower, Jaminski, Schieber, Zimmerle and Sethi1998), with the chemocline very close to the sediment-water interface (Jones & Manning, Reference Jones and Manning1994) and no significant differences between MSGE and non-MSGE (Figure 8a and Supplementary Table 2). The alternative use of the U and Mo enrichment factors (Algeo & Tribovillard, Reference Algeo and Tribovillard2009;Algeo & Li, Reference Algeo and Li2020) yields similar results (Figure 8b). The absence of covariation between both factors and the consistently low absolute values of MoEF and UEF for all samples, except for one (UEF = 7.32), support the interpretation of generally oxic marine conditions, with the redox chemocline positioned near the water-sediment interface (Algeo & Tribovillard, Reference Algeo and Tribovillard2009). For full discussion on redox condition of IPB sulphide-hosting environments see Sáez et al. (Reference Sáez, Moreno, González and Almodóvar2011). Although the precise extension of the oxygen-depleted water column is not provided by these geochemical data, the abundance of organic-walled microphytoplankton, including M. mosesii, in all samples suggests a vertical zonation pattern with oxygenated water at least in the upper photic zone. Both acritarch cysts and prasinophyte phycomata, the two microphytoplankton groups reported in the studied samples, are generated by the encystment of motile aerobic organisms that, for comparison with modern analogues, require a photic and oxygenated habitat for living (Wong et al. Reference Wong, Raven, Aldunate, Silva, Gaitán-Espitia, Vargas, Ulloa and Von Dassow2023).
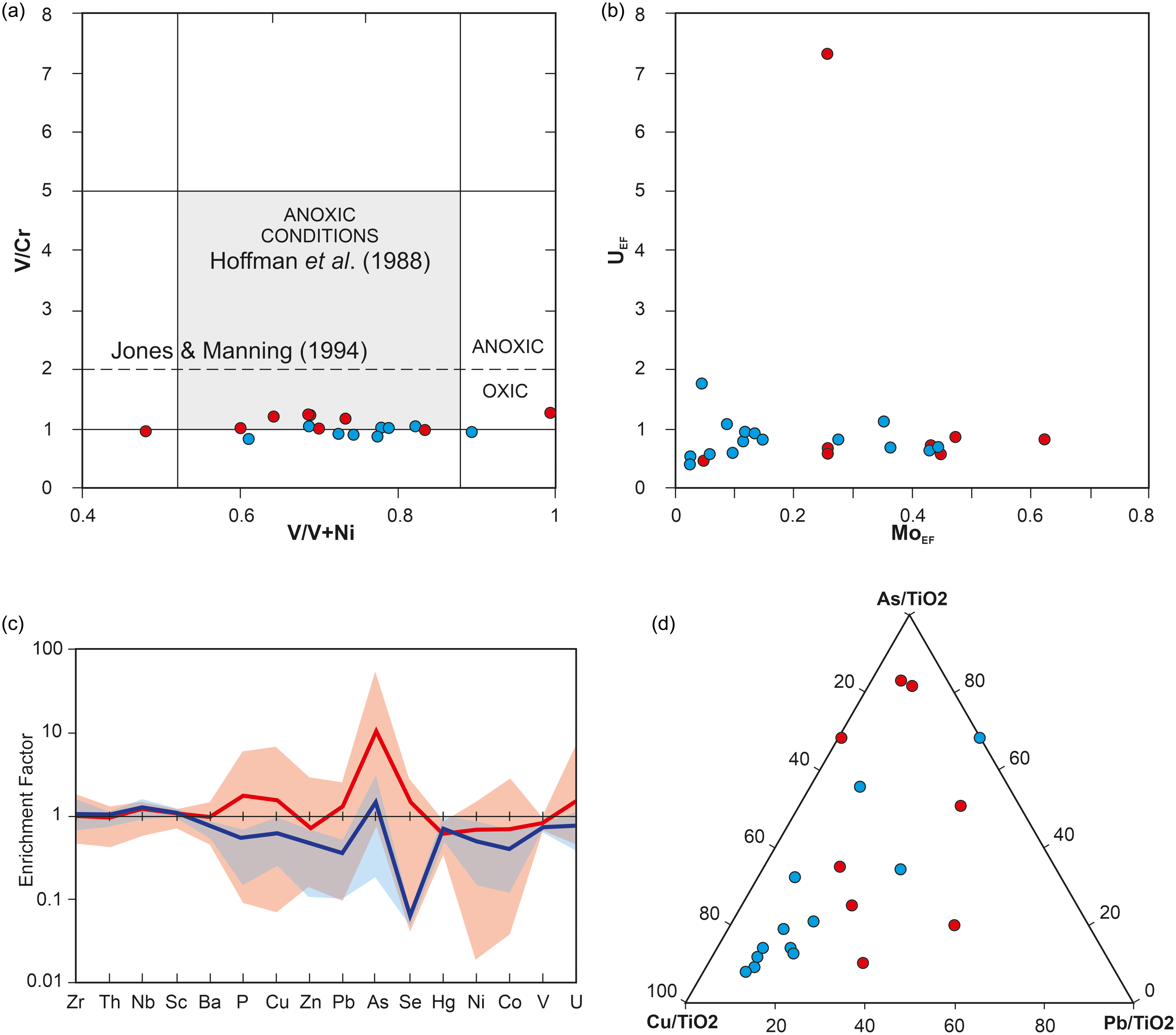
Figure 8. (a) Bivariate plot of the black shale samples from MSGE (red circles) and non-MSGE (blue circles) in the diagram V/Cr vs V/(V+Ni). Limits of environmental conditions after the Hoffman et al. (Reference Hoffman, Akgeo, Maynard, Joachimski, Hower, Jaminski, Schieber, Zimmerle and Sethi1998) and Jones and Manning (Reference Jones and Manning1994); (b) Diagram UEF vs MoEF; (c) PAAS-normalized spider-like diagrams for maximum, minimum and average values (bold lines) of black shale samples. Red and blue lines represent samples from MSGE and non-MSGE, respectively; (d) Ternary diagram showing the relative proportion of Cu-As-Pb normalized to TiO2. Red and blue circles for MSGE and non-MSGE, respectively.
The comparison of spider-like diagrams for black shales from the two environment sceneries considered in this study shows strong positive anomaly for As and Se in MSGE. Minor anomalies for P, Cu, Zn and Pb are also noticeable (Figure 8c). Analytical values for Cu, Pb and As normalized to TiO2 are presented in Figure 8d. It shows that all but two samples from non-MSGE form a cluster near the Cu corner, whereas shales from MGSE are dispersed and relatively enriched in As and Pb.
In the IPB, the generation of sulfide deposits was associated with highly efficient hydrothermal systems (Sáez et al. Reference Sáez, González, Donaire, Toscano, Yesares, Almodóvar and Moreno2024). High-temperature hydrothermal mineralizing fluids co-transported reduced sulphur and metals from deeper to near-surface zones. Under this scenario, the occurrence of Cu, As and Pb in MSGE seafloor, along with the other metals that ultimately contributed to the formation of the massive sulphide deposits, would be expected.
In non-polluted marine environments, Cu is generally used as proxy for bioproductivity (Tribovillard et al. Reference Tribovillard, Riboulleau, Lyons and Baudin2004; Riquier et al. Reference Riquier, Tribovillard, Averbuch, Devleeschouwer and Riboulleau2006), while As and Pb are considered highly poisonous for living organisms (Davies, Reference Davies1979; Sharma & Sohn, Reference Sharma and Sohn2009).
5. Discussion
The conjugate analysis of the three techniques employed provides information about the interaction of M. mosesii with their hosting environment. Although the morphological variability detected here is linked with the growth trend previously accounted for this species (González, Reference González2009), specimens reported in MSGE are systematically smaller and lighter than those from non-MSGE. Besides, their external wall is enriched in As, a poisoned element more abundant in MSGE than in non-MSGE MSGE that is common in IPB massive sulphide and stockwork mineralization in the form of arsenopyrite and tennantite (Almodóvar et al. Reference Almodóvar, Yesares, Sáez, Toscano, González and Pons2019). This indicates that cysts of M. mosesii sank after encystment, maturating at the bottom of the different sub-basins. There, the disparate environmental conditions conditioned the cyst maturation.
In living, cyst-producing dinoflagellates, the intraspecific morphological variability has been largely associated with changes in temperature, salinity, nutrient levels or pollutants (Dale, Reference Dale, Jansonius and McGregor1996; Ellegaard et al. Reference Ellegaard, Lewis and Harding2002, Reference Ellegaard, Dale, Mertens, Pospelova, Ribeiro, Weckström, Saunders, Gell and Skilbeck2017; Pospelova et al. Reference Pospelova, Chmura, Boothman and Latimer2002, Reference Pospelova, Chmura, Boothman and Latimer2005). In the fossil record, morphological perturbations of dinocysts and acritarchs have been also associated with local changes in salinity (Dybkjær, Reference Dybkjær2004), but more commonly with global factors such as fluctuations in the C cycle (Delabroye et al. Reference Delabroye, Munnecke, Servais, Vandenbroucke and Vecoli2012; Munnecke et al. Reference Munnecke, Delabroye, Servais, Vandenbroucke and Vecoli2012) or expansion of oceanic anoxic events (Vandenbroucke et al. Reference Vandenbroucke, Emsbo, Munnecke, Nuns, Duponchel, Lepot, Quijada, Paris, Servais and Kiessling2015). Nevertheless, the variability detected in acritarchs and dinoflagellates, either living or fossil, is understood as the interaction of their parent flagellate cells with the surrounding environment (Kremp et al. Reference Kremp, Rengefors and Montresor2009; Lundholm et al. Reference Lundholm, Ribeiro, Andersen, Koch, Godhe, Ekelund and Ellegaard2011). During the non-flagellate phase, the metabolic activity drastically decreases, remaining, in most cases, at the expense of internal carbohydrate reserves (Binder & Anderson, Reference Binder and Anderson1990). Thus, the resting cysts of acritarchs and dinoflagellates virtually operate as closed systems, sensitive to variations in temperature, light and redox conditions (Anderson et al. Reference Anderson, Stock, Keafer, Nelson, Thompson, McGillicuddy, Keller, Matrai and Martin2005; Brosnahan et al. Reference Brosnahan, Fischer, López, Moore and Anderson2020), but not interacting or interacting minimally with their hosting environment.
In the case of living or fossil prasinophytes, studies about intraspecific morphological variations and differential growth rates are very scarce. The available data suggest that, although the morphology of the prasinophytes cyst, called phycomata, can be governed by global climatic factors (Spiridonov et al. Reference Spiridonov, Venckutë-Aleksienë and Radzevičius2017), it must be also influenced by the environmental conditions prevailing at maturation sites (Boalch & Mommaerts, Reference Boalch and Mommaerts1969). There, the substantial phycomata enlargement (Wiebe et al. Reference Wiebe, Remsen and Vaccaro1974; Parke et al. Reference Parke, Boalch, Jowett and Harbour1978; Colbath, Reference Colbath1983; Knoll et al. Reference Knoll, Swett and Mark1991; Tyson, Reference Tyson1995), which in current prasinophytes can represent an increase of up to 5000 times the size of the precursor flagellate cells (Tappan, Reference Tappan1980), requires of an extracellular energetic source (Pazio, Reference Pazio2016).
On the other hand, the effects of As on modern phytoplankton have been studied in detail. Although with different efficiency, these organisms are capable of bio-uptaking inorganic As, generally as arsenate (III), and subsequently reduce it to arsenite (IV) in order to form more complex and less toxic methylated organic compounds that are then excreted as a detoxification mechanism (Neff, Reference Neff1997; Yamaoka et al. Reference Yamaoka, Takimura, Fuse and Murakami1999; Goessler et al. Reference Goessler, Lintschinger, Szakova, Mader, Kopecky, Douch and Irgolic1997; Caumette et al. Reference Caumette, Koch, Estrada and Reimer2011; Giovannoni et al. Reference Giovannoni, Halsey, Saw, Muslin, Suffridge, Sun, Lee, Moore, Temperton and Noell2019). They are even capable of assimilating small proportions of inorganic As. However, when they are exposed to anomalously high As concentrations, their maturation capacity can be affected and even their lethality increases (Planas & Healey Reference Planas and Healey1978). This behaviour regarding As refers exclusively to non-cyst-forming species or to the flagellate phase of cyst-forming phytoplanktonic organisms. The drastic metabolic reduction in their cyst phase minimizes their element assimilation and their potential infection. This explains the absence of information in the available literature on the effect that As, or any other element, can produce on dinoflagellate and acritarch resting cysts. There are also no studies to our knowledge on the effect of the environment on current prasinophyte phycomata, despite in this case, its huge enlargement suggests metabolic activity beyond the consumption of internal carbohydrate reserves.
In the absence of comparative studies and considering the environmentally induced behaviour of M. mosesii here detected, we hypothesize that this species established an anaerobic interchange with the surrounding oxygen-depleted environment during cyst maturation and that As fixation was a by-product of such metabolic process. During maturation, the metabolic activity responsible for As assimilation gradually gained importance. This explains the different As concentrations detected among specimens of a given sample (Figure 7). In non-MSGE, the cysts of M. mosesii also contain As, although in minor proportion. This is explained by the ubiquitous occurrence of As in the marine realm, particularly in oxygen-depleted environments (Leventhal, Reference Leventhal1991; Bodin et al. Reference Bodin, Godet, Matera, Steinmann, Vermeulen, Gardin, Adatte, Coccioni and Föllmi2006; Liu & Cai, Reference Liu and Cai2010). When cysts reached an optimum level of maturation, regardless of the level of As accumulated, they moved up in the water column favoured by near-bottom and/or upwelling currents, and finally dispersed their offspring in well-oxygenated near-surface waters. Supporting this hypothesis is the fact that cyst-forming phytoplankton requires oxygenated environments for excystment and germination (Anderson et al. Reference Anderson, Taylor and Armbrust1987; Kremp & Anderson, Reference Kremp and Anderson2000; Ellegaard et al. Reference Ellegaard, Dale, Mertens, Pospelova, Ribeiro, Weckström, Saunders, Gell and Skilbeck2017), and that specimens of M. mosesii having excystment structures, i.e., those that resuspended to the photic zone and settled on the bottom again, are in much smaller proportion than non-excysted cysts. The equatorial solid pads in this species (Figures 3 and 5) could have been used as energy storage devices or as structures within a floating/sinking system. Their consumption or detachment, joined with the development and progressive enlargement of the equatorial, hollow, transparent bladders that characterize the latest growth stages in this species (González, Reference González2009), favoured the final resuspension of mature cysts. The elevated concentration of As in MSGE decreased the metabolic interchange with the surrounding medium or accelerated the cysts maturation, and with it their resuspension, in order to attenuate, or diminish, the exposure to a hostile environment. The reduced equatorial diameter and small amount of pad-bearing cysts were the consequences of this process.
The common to elevated occurrence of M. mosesii cysts in most MSGE samples suggests that such adaptative mechanism was effective. However, the quantitative analysis of the relative proportion of acritarchs, prasinophyte phycomata and M. mosesii specimens (Figure 9) shows that MSGE samples are mostly dominated by acritarchs whereas non-MSGE samples are mostly dominated by M. mosesii. In addition, the percentage of this species is most fluctuating in MSGE than in non-MSGE samples. This means that, although M. mosesii can be adapted to pollutant conditions, they germinate more abundantly in non-stressed environments.
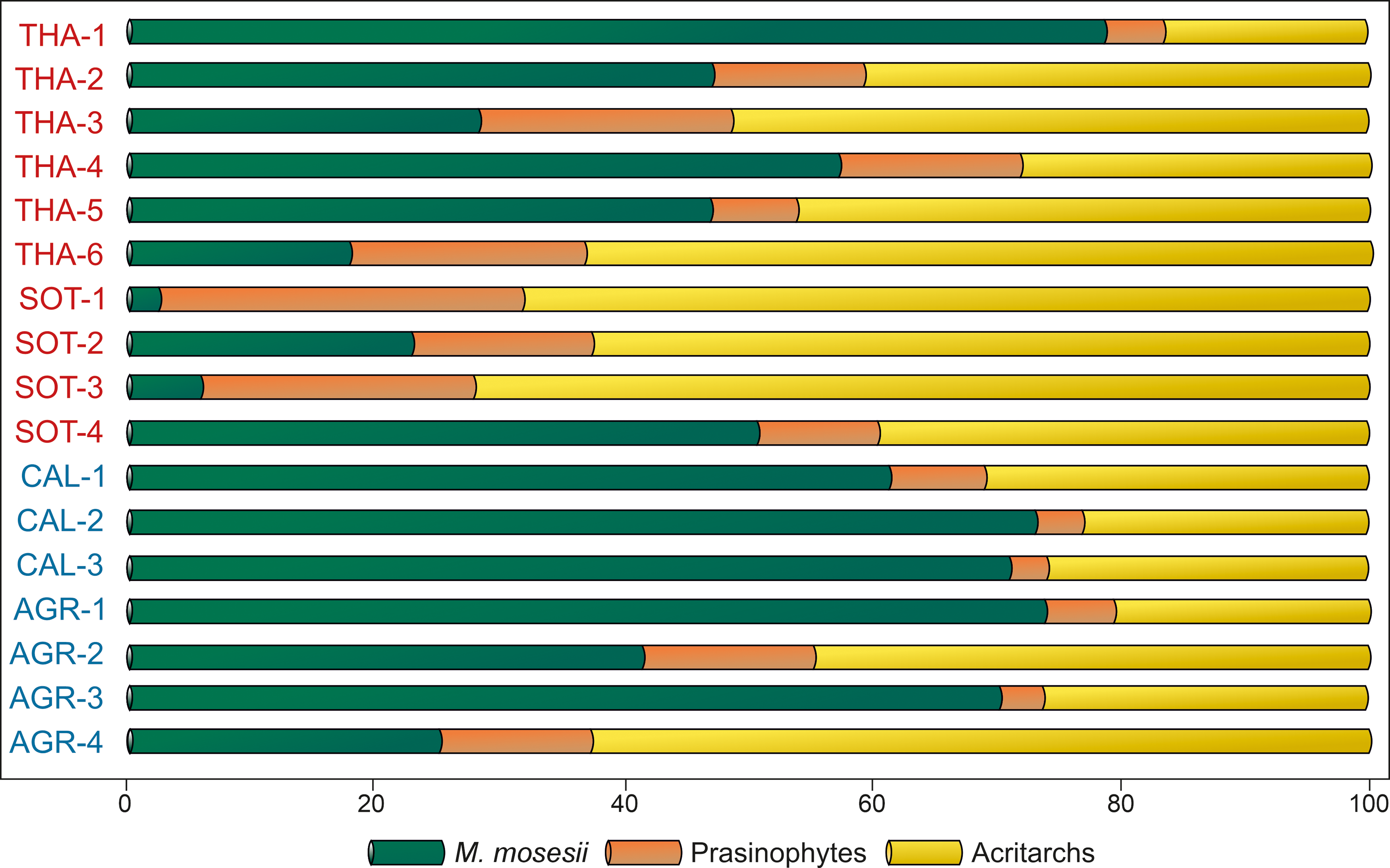
Figure 9. Quantitative analysis of the phytoplankton recovered in the studied samples (in percentages). Samples in red from MSGE. Samples in blue from non-MSGE. Location of samples is indicated in Figure 1.
Regardless of whether cyst size reduction in M. mosesii is an adaptive mechanism or a limitation resulting from growing under hostile conditions, a causal relationship between cyst size and hosting environment is clearly established. This means that, in the IPB, the biometric analysis of M. mosesii specimens provides an effective tool for distinguishing late Devonian black shales sequences associated with massive sulphides from barren sequences (without massive sulphides). To date, the contribution of palynology to the study of the IPB basin has primarily focused on the detailed biostratigraphic analysis of shale sequences belonging to its three chronostratigraphic units, with special emphasis on the VSC sequences hosting the massive sulphides and the coeval underlaying PQ sequences cut by stockwork type mineralization (i.e., Pereira et al. Reference Pereira, Sáez, Pons, Oliveira and Moreno1996, 2004, Reference Pereira, Matos, Solá, Batista, Salgueiro, Rosa, Albardeiro, Mendes, Morais, de Oliveira, Pacheco, Araújo, Castelo Branco, Neto, Lains Amaral, Inverno and Oliveira2021, Reference Pereira, Matos, Mendes, Solá, Albardeiro, Morais, Araújo, Pacheco and Oliveira2023; González et al. Reference González, Moreno and Sáez2002, González et al., Reference González, Moreno and Santos2006; Mendes et al. Reference Albardeiro, Matos, Mendes, Solá, Pereira, Morais, Salgueiro, Pacheco, Araújo and Oliveira2020, Reference Mendes, Pereira, Matos, Albardeiro, Morais and Araújo2024; Sáez et al. 2007). The present study goes a step further by aiding in the identification of potential targets for sulphide exploration in the region, paying special attention to the massive sulphide and stockwork-type mineralization hosted by black shales.
Finally, in terms of suprageneric affiliation, M. mosesii, and for extension the genus Maranhites (Brito 1965) emend González Reference González2009, cannot be unquestionably assigned to the Prasinophyceae Class due to the absence of morphologically comparable extant counterparts. However, its preference for oxygen-depleted environments and its active metabolism during the cyst phase, unreported in living and fossils acritarch and dinoflagellates, reinforces the hypothesis by Tappan (Reference Tappan1980), Colbath and Grenfell (Reference Colbath and Grenfell1995) and Lé Hérissé et al. (Reference Le Hérissé, Dorning, Mullins and Wicander2009) by which Maranhites may have prasinophyte affinity.
6. Conclusions
The latemost Devonian palynological assemblages from the IPB are unusually enriched in the microphytoplankton species M. mosesii. This species is abundant in both MSG and non-MSGE environments. The geochemistry of the shales recovered in MSG and non-MSGE environments indicates that both habitats are characterized by oxygen-depleted seafloor conditions and that most of the MSGE samples were enriched in pollutants, including As and Pb. The specimens of M. mosesii reported in MSGE were smaller and lighter than those from non-MSGE. Besides, the MSGE specimens depicted anomalous high values of As on their outer walls. This indicates that cysts of this species sank after encystment and were affected during maturation by disparate seafloor environmental conditions. The abundance of M. mosesii in both groups of environments also suggests that this species implemented during its growing cyst phase a successful adaptive mechanism to oxygen-depleted conditions that might involve anaerobic metabolic interchange with the surrounding media and high level of tolerance to stressors.
The multi-analytical analysis employed in this study, which involves (1) biometry of microphytoplankton fossils, (2) TOFSIMS imaging and surface analysis of selected specimens and (3) host rock geochemistry, has resulted useful for unravelling the interrelation of metabolically active cysts with its surrounding seafloor environment. The causal relationship established between cyst size and environmental conditions allows the biometry of M. mosesii to be viewed as a promising prospective vector for massive sulphide and stockwork-type deposits in the IPB.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0016756825100034
Acknowledgements
The original manuscript was improved thanks to the suggestions and comments of five anonymous reviewers.
Financial support
This research was funded by the CICYT (Spain) Research Project CGL2016-79204-R and the Research Group THARSIS RNM 198-PAI (Junta de Andalucía).











