Persuasive interventions, such as prospective audit and feedback, are recommended strategies for antimicrobial stewardship programs (ASPs). These interventions aim to optimize antibiotic use by direct interaction with the prescriber after the antibiotic is prescribed. However, the clinical microbiology laboratory is an important partner in initiatives to improve antibiotic use and has the potential to influence antibiotic prescribing before the prescription is written. The Infectious Diseases Society of America (IDSA) Guidelines for Antimicrobial Stewardship recommend that ASPs implement selective or cascade microbiology reporting to guide prescribers to select an appropriate antimicrobial agent based on a limited list of reported options.Reference Barlam, Cosgrove and Abbo1 However, given the lack of strong supportive evidence for this strategy, it is considered a weak recommendation. The Clinical Laboratory Standards Institute (CLSI) also recommends the use of selective reporting but suggests that individual decisions are best made by the institution’s infectious diseases, microbiology, and pharmacy team.2
Behavioral approaches to guide decision making through the strategic placement of choice architecture while still maintaining prescriber autonomy are called nudging strategies. Nudging can be used in the clinical microbiology laboratory given the need for high-yield interdisciplinary interventions to improve the initiation, selection, and/or duration of antibiotic use in all healthcare settings. In the context of susceptibility test result reports, nudging can include (1) presenting 1 or more default options that are more desirable than other options and masking nondesirable options; (2) framing recommendations by adding comments or context to guide decision making; and (3) presenting desired options at eye level by keeping desired choices at the top of the list or emphasizing the text for desired agents.Reference Katchanov, Kluge, MacKenzie and Kaasch3 Despite recent interest in this approach, the type and breadth of microbiology nudging strategies studied in the literature has not yet been systematically evaluated. We conducted a scoping review instead of a systematic review because nudging in the context of the microbiology result reporting is not well defined, and we expected that the literature yield would be sparse and heterogeneous. Unlike systematic reviews or meta-analyses, scoping reviews are designed to provide an overview of a broad range of literature rather than appraise or quantitatively synthesize data on a focused topic. Scoping reviews use a systematic and rigorous approach to map the literature and to identify the main concepts and opportunities for future research.Reference Tricco, Lillie and Zarin4, Reference Arksey and O’Malley5 We sought to conduct a scoping review to identify the evidence and gaps that exist for microbiology nudging strategies. The following research question was formulated: What evidence exists for the use of nudging strategies in microbiology to improve antimicrobial use?
Methods
Protocol
This scoping review was conducted as an initial step to a broader research program entitled Nudging in MicroBiology Laboratory Evaluation (NIMBLE), to investigate opportunities to improve nudging in microbiology reporting. A scoping review protocol was developed a priori involving a team composed of a medical microbiologist, infectious diseases physicians, infectious diseases pharmacists, and a medical librarian. A Participant-Intervention-Comparator-Outcome (PICO) framework was used to guide the search strategy and article selection. The protocol is available upon request from the corresponding author. The PRISMA Extension for Scoping Reviews (PRISMA-ScR) recommendations were followed.Reference Tricco, Lillie and Zarin4
Eligibility
All inpatient, outpatient, adult, and pediatric patient populations were eligible for inclusion. All real-life microbiology nudging interventions included, but were not limited to, default choice, framing, and eye-level nudging strategies. Case vignettes, surveys, and animal studies were excluded. We placed no restriction on comparison group or study design, nor did we restrict outcomes evaluated in each study.
Information sources and search strategy
A medical librarian formulated a search for studies in the following databases: Ovid MEDLINE: Epub Ahead of Print, In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE, 1946–August 2018, Embase Classic+Embase 1947–August 2018, PsycINFO 1806–August 2018, and All EBM Reviews 2005–August 2018. The main broad concepts in the search included microbiology reports, antimicrobial treatment, and nudging strategies. The search strategy was peer reviewed by a second medical librarian and further refined. The search was conducted on August 27, 2018. Search strategies for each published literature database are available in Appendix A. Review of each article’s reference list was also undertaken.
We conducted a grey literature search on December 7, 2018, for conference proceedings from the following conferences: IDWeek (Infectious Diseases Society of America), SHEA (Society for Healthcare Epidemiology of America), ICAAC (Interscience Conference on Antimicrobial Agents and Chemotherapy), ASM-Microbe (American Society for Microbiology), AMMI-Canada CACMID (Association for Medical Microbiology and Infectious Diseases Canadian Association for Clinical Microbiology and Infectious Diseases), and ECCMID ( European Society of Clinical Microbiology and Infectious Diseases).
Study selection process
Search results and selection criteria were incorporated into Colandr online systematic review software, (Science for Nature and People Partnership, Conservation International, and DataKind, 2018). Two independent reviewers (B.L. and L.M.) participated in title and abstract screening and full-text screening in duplicate. Screening was performed in a blinded fashion, and disagreements were resolved by consensus. A third reviewer was available in cases in which consensus could not be reached.
Data items and data collection process
A predefined list of variables for data collection was agreed upon prior to data extraction: citation, year, country, healthcare setting, patient population, study design, sample size, organisms studied, antibiotics studied, source of samples studied , nudging strategy, other ASP strategies reported, follow up period, outcomes reported, author conclusions. Data were charted by a single reviewer (B.L.) and were verified by a second reviewer (L.M.).
Results
After duplicates were removed, a total of 1,342 articles were identified via database searching. Three additional sources were identified from reference list searching, plus 1 additional reference from the grey literature search, for a total of 1,346 potential articles for inclusion. Based on title and abstract screening, 1,325 articles were excluded. Of 21 articles that underwent full-text screening, 6 were excluded. Excluded studies were case vignettes or surveys (n = 2), did not define a clear nudging intervention (n = 3), or was a study protocol (n = 1). After exclusion, 15 studies met eligibility for inclusion in the scoping review.Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6–Reference Brodowy, Guglielmo, York, Herfindal and Brooks20 Figure 1 shows the flow diagram for study inclusion.
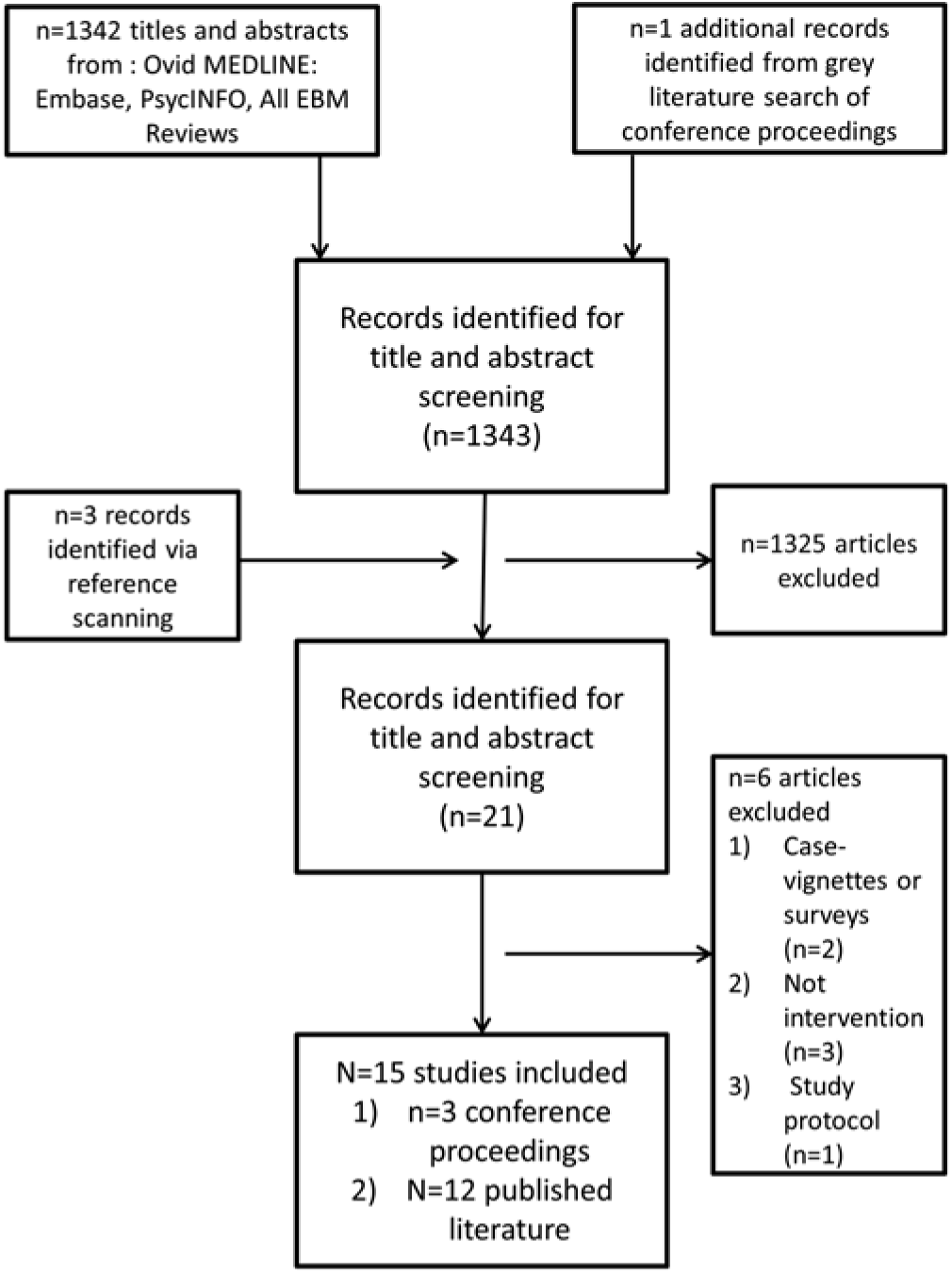
Fig. 1. Scoping review flow diagram. Details of study selection throughout the phases of the scoping review, including studies included via published and grey literature as well as studies excluded.
Characteristics of studies included
Of the included studies, the most common country of origin was the United States (n = 6)Reference Liao, Rhodes and Sopirala14, Reference Mcbride, Schulz, Fox, Dipoto, Sippel and Osterby15, Reference Musgrove, Kenney and Kendall17, Reference Steffee, Morrell and Wasilauskas18, Reference Brodowy, Guglielmo, York, Herfindal and Brooks20, followed by Canada (n = 3),Reference Daley, Garcia, Inayatullah, Penney and Boyd9, Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference Leis, Rebick and Daneman13 the United Kingdom (n = 3),Reference Barnes7, Reference McNulty, Lasseter and Charlett16, Reference Tan, McNulty, Charlett, Nessa, Kelly and Beswick19 Ireland (n = 1),Reference Cunney, Aziz, Schubert, McNamara and Smyth8 Singapore (n = 1),Reference Foo, Tan, Loh, Chung, Chew and Bagdasarian10 and Saudi Arabia (n = 1).Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6 Most studies (n = 10) were published recently (2010–2018),Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Daley, Garcia, Inayatullah, Penney and Boyd9–Reference Musgrove, Kenney and Kendall17 most studies evaluated the inpatient population (n = 13),Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6–Reference Mcbride, Schulz, Fox, Dipoto, Sippel and Osterby15, Reference Musgrove, Kenney and Kendall17, Reference Steffee, Morrell and Wasilauskas18, Reference Brodowy, Guglielmo, York, Herfindal and Brooks20 and the remaining 2 studiesReference McNulty, Lasseter and Charlett16, Reference Tan, McNulty, Charlett, Nessa, Kelly and Beswick19 focused on primary care. Study designs included a randomized controlled trial (n = 1),Reference Daley, Garcia, Inayatullah, Penney and Boyd9 pre- and postintervention with control (n = 3),Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference Leis, Rebick and Daneman13, Reference Musgrove, Kenney and Kendall17 pre- and postintervention without control (n = 7),Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Foo, Tan, Loh, Chung, Chew and Bagdasarian10, Reference Johnson, Patel, King and Maslow11, Reference Liao, Rhodes and Sopirala14, Reference Mcbride, Schulz, Fox, Dipoto, Sippel and Osterby15, Reference Steffee, Morrell and Wasilauskas18 and retrospective cohort (n = 4).Reference Barnes7, Reference Cunney, Aziz, Schubert, McNamara and Smyth8, Reference Tan, McNulty, Charlett, Nessa, Kelly and Beswick19, Reference Brodowy, Guglielmo, York, Herfindal and Brooks20 Sample size, in terms of number of patients or isolates, ranged from 72Reference Steffee, Morrell and Wasilauskas18 to 1,522Reference Liao, Rhodes and Sopirala14 (median, 179), and was not specified in 4 studies.Reference Foo, Tan, Loh, Chung, Chew and Bagdasarian10, Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference McNulty, Lasseter and Charlett16, Reference Tan, McNulty, Charlett, Nessa, Kelly and Beswick19 Table 1 displays a summary of study characteristics, nudging strategies employed, and outcomes measured.
Table 1. Characteristics of Nudging in Microbiology Studies

Note. MRSA, methicillin-resistant Staphylococcus aureus; DDD, defined daily dose; UTI, urinary tract infection.
Nudging strategies employed
Anatomical samples of interest for microbiology report modification in order of most common to least common were all samples (no restriction on anatomical site) (n = 6),Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Foo, Tan, Loh, Chung, Chew and Bagdasarian10, Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference Liao, Rhodes and Sopirala14, Reference Steffee, Morrell and Wasilauskas18, Reference Brodowy, Guglielmo, York, Herfindal and Brooks20 urine (n = 5),Reference Barnes7, Reference Daley, Garcia, Inayatullah, Penney and Boyd9, Reference Leis, Rebick and Daneman13, Reference McNulty, Lasseter and Charlett16, Reference Tan, McNulty, Charlett, Nessa, Kelly and Beswick19 respiratory (n = 2),Reference Mcbride, Schulz, Fox, Dipoto, Sippel and Osterby15, Reference Mcbride, Schulz, Fox, Dipoto, Sippel and Osterby15, Reference Musgrove, Kenney and Kendall17 blood (n = 1),Reference Johnson, Patel, King and Maslow11and mixed (n = 1).Reference Cunney, Aziz, Schubert, McNamara and Smyth8 Targeted organisms included all (n = 7),Reference Barnes7–Reference Foo, Tan, Loh, Chung, Chew and Bagdasarian10, Reference Leis, Rebick and Daneman13, Reference McNulty, Lasseter and Charlett16, Reference Tan, McNulty, Charlett, Nessa, Kelly and Beswick19 gram-negative bacteria (n = 5),Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Johnson, Patel, King and Maslow11, Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference Liao, Rhodes and Sopirala14, Reference Brodowy, Guglielmo, York, Herfindal and Brooks20methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (n = 2),Reference Mcbride, Schulz, Fox, Dipoto, Sippel and Osterby15, Reference Musgrove, Kenney and Kendall17 and gram-positive bacteria (n = 1).Reference Steffee, Morrell and Wasilauskas18, Reference Brodowy, Guglielmo, York, Herfindal and Brooks20
A default choice nudging strategy was employed in most studies (n = 13).Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6–Reference Liao, Rhodes and Sopirala14, Reference McNulty, Lasseter and Charlett16, Reference Steffee, Morrell and Wasilauskas18–Reference Brodowy, Guglielmo, York, Herfindal and Brooks20 In these studies, selective or cascade antimicrobial susceptibility reporting were the most common approaches (n = 10).Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Cunney, Aziz, Schubert, McNamara and Smyth8, Reference Foo, Tan, Loh, Chung, Chew and Bagdasarian10–Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference Liao, Rhodes and Sopirala14, Reference McNulty, Lasseter and Charlett16, Reference Steffee, Morrell and Wasilauskas18–Reference Brodowy, Guglielmo, York, Herfindal and Brooks20 Studies either evaluated the impact of masking (n = 8)Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Cunney, Aziz, Schubert, McNamara and Smyth8, Reference Johnson, Patel, King and Maslow11, Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference Liao, Rhodes and Sopirala14, Reference McNulty, Lasseter and Charlett16, Reference Tan, McNulty, Charlett, Nessa, Kelly and Beswick19, Reference Brodowy, Guglielmo, York, Herfindal and Brooks20 or unmasking (n = 2)Reference Foo, Tan, Loh, Chung, Chew and Bagdasarian10, Reference Steffee, Morrell and Wasilauskas18 of antimicrobial agents. Although the impact of selective reporting for a broad range of antibiotics has been evaluated in several studies, fluoroquinolonesReference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Barnes7, Reference Foo, Tan, Loh, Chung, Chew and Bagdasarian10, Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference Tan, McNulty, Charlett, Nessa, Kelly and Beswick19 and broad-spectrum β-lactam agentsReference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Barnes7, Reference Johnson, Patel, King and Maslow11, Reference Liao, Rhodes and Sopirala14, Reference McNulty, Lasseter and Charlett16, Reference Brodowy, Guglielmo, York, Herfindal and Brooks20 were the most typical targets of selective reporting strategies with the goal of reducing overuse of these agents.
Two studies aimed to reduce overtreatment of asymptomatic bacteriuria.Reference Daley, Garcia, Inayatullah, Penney and Boyd9, Reference Leis, Rebick and Daneman13 These studies both evaluated a “modified” reporting approach that replaced the typical culture with a susceptibility report with a standardized comment to contact microbiology for the results if clinically warranted. These studies incorporated aspects of both default-choice nudging strategies (ie, concealing results) and framing strategies (ie, comments on reports suggesting the likelihood of asymptomatic bacteriuria).
A framing approach was used in 2 studies.Reference Mcbride, Schulz, Fox, Dipoto, Sippel and Osterby15, Reference Musgrove, Kenney and Kendall17 Authors evaluated the impact of adding additional comments to respiratory cultures where commensal flora were identified, and they were explicit about the lack of certain pathogens (ie, methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas spp). In these cases, a statement of “no MRSA/no Pseudomonas” was added to the report to discourage broad-spectrum antibiotic use and encourage de-escalation of therapy.
No included studies evaluated the impact of eye-level nudging strategies in microbiology reports.
Potential for confounding
Of the 14 nonrandomized studies included in this review, only 5 reported on other activities coexisting at the time of nudging intervention implementation. Most of these studies reported ongoing ASP activities or education.Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Barnes7, Reference Johnson, Patel, King and Maslow11, Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference Musgrove, Kenney and Kendall17
Outcomes measured
A primary outcome was prespecified in 7 studies.Reference Daley, Garcia, Inayatullah, Penney and Boyd9–Reference Liao, Rhodes and Sopirala14, Reference Musgrove, Kenney and Kendall17 All studies reported an outcome measure of antimicrobial use including prescribing or utilization measures (eg, defined daily doses) (n = 10),Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6–Reference Cunney, Aziz, Schubert, McNamara and Smyth8, Reference Foo, Tan, Loh, Chung, Chew and Bagdasarian10, Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference Liao, Rhodes and Sopirala14–Reference McNulty, Lasseter and Charlett16, Reference Steffee, Morrell and Wasilauskas18, Reference Tan, McNulty, Charlett, Nessa, Kelly and Beswick19 appropriateness (n = 7),Reference Barnes7–Reference Foo, Tan, Loh, Chung, Chew and Bagdasarian10, Reference Leis, Rebick and Daneman13, Reference Steffee, Morrell and Wasilauskas18, Reference Brodowy, Guglielmo, York, Herfindal and Brooks20de-escalation rate (n = 2),Reference Johnson, Patel, King and Maslow11, Reference Musgrove, Kenney and Kendall17 or cost (n = 1).Reference Daley, Garcia, Inayatullah, Penney and Boyd9 Twelve studies reported an improvement in targeted antibiotic use associated with a nudging approach,Reference Daley, Garcia, Inayatullah, Penney and Boyd9–Reference Brodowy, Guglielmo, York, Herfindal and Brooks20 and 2 studies showed mixed results.Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Cunney, Aziz, Schubert, McNamara and Smyth8 Another found no improvement.Reference Barnes7
Also, 4 studies evaluated the association of nudging strategy with subsequent antimicrobial resistance,Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference McNulty, Lasseter and Charlett16, Reference Musgrove, Kenney and Kendall17 and 2 studies noted overall improvement in selected antimicrobial resistance rates.Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference Musgrove, Kenney and Kendall17 Other safety outcomes studied included C. difficile infection, length of hospital stay, readmission rate, and mortality. One study found an improvement in C. difficile infection ratesReference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6 after the implementation of a selective reporting policy. All other studies found no change in these outcome measures,Reference Johnson, Patel, King and Maslow11, Reference Liao, Rhodes and Sopirala14, Reference Musgrove, Kenney and Kendall17 suggesting that nudging studies are not likely to be associated with adverse outcomes.
Discussion
We conducted a scoping review of nudging in microbiology reports and found 15 relevant but heterogeneous studies, most of which focused on a default-reporting nudging strategy. The bulk of this literature was published in the current decade, which indicates a recent rapid growth of interest in nudging approaches in microbiology.
Selective or cascade reporting, a strategy in which preferable default antibiotic options are displayed on the report and less desirable options are suppressed, was the most common strategy evaluated. Although most studies have shown a positive association with improved prescribing practices, the lack of prospective, randomized data in this literature as well as lack of comprehensive evaluation of clinical outcomes prevent this approach from becoming more widespread and standardized across facilities. Additionally, several studies have noted a shift from one antibiotic to another,Reference Al-Tawfiq, Momattin, Al-Habboubi and Dancer6, Reference Langford, Seah, Chan, Downing, Johnstone and Matukas12, Reference McNulty, Lasseter and Charlett16 a concept termed “squeezing the balloon.”Reference Peterson21 Generally, nudges result in a shift to a more desirable antibiotic agent, but antimicrobial stewards need to consider the potential unintended consequences of shifts in usage and subsequent resistance patterns.
A large cross-sectional survey on selective reporting policies in Europe found vast inconsistencies across and within countries in the implementation of selective reporting of susceptibility results.Reference Pulcini, Tebano and Mutters22 Selective reporting of antimicrobial susceptibility results was implemented in 31% of countries, with only 3 national health authorities endorsing it as the standard of care. Lack of detailed guidelines, poor system support to implement and automate such a strategy, insufficient human resources, and lack of priority were cited as barriers to advancing this practice.
Although not specifically evaluated in this review, several case-vignette studies of nudging strategies have been performed and have also shown an impact on antibiotic selection and improvement in appropriateness of therapy.Reference Bourdellon, Thilly, Fougnot, Pulcini and Henard23–Reference Papanicolas, Nelson and Warner25 The case-vignette approach allows microbiologists and ASP teams to fine tune selective reporting to different clinical scenarios and contextual factors unique to each region in a test environment. This step may be helpful before moving forward to prospective studies.
Modified reporting, specifically concealing positive culture results, has shown promise in reducing unnecessary treatment of asymptomatic bacteriuria in noncatheterized patients.Reference Daley, Garcia, Inayatullah, Penney and Boyd9, Reference Leis, Rebick and Daneman13 The only randomized controlled trialReference Daley, Garcia, Inayatullah, Penney and Boyd9 in this scoping review evaluated this approach; which presents an opportunity to reduce overall antibiotic exposure for asymptomatic bacteriuria, a common condition seen in acute and long-term care populations. Preventing unnecessary treatment will likely present the highest yield antimicrobial stewardship opportunity for nudging in the microbiology laboratory. Our scoping review identifies a number of important gaps in the research that may require further evaluation to improve the scope and quality of nudging in microbiology report strategies. Given that most antibiotic use occurs in the community setting, advances in antimicrobial stewardship approaches should be developed for application to this group. Only 2 of 15 studies in the scoping review focused on an outpatient population,Reference McNulty, Lasseter and Charlett16, Reference Tan, McNulty, Charlett, Nessa, Kelly and Beswick19 revealing a gap and potential opportunity for future research. Notably, only 2 studies exclusively evaluated a framing strategy, in which comments are added to the report to guide decision making,Reference Mcbride, Schulz, Fox, Dipoto, Sippel and Osterby15, Reference Musgrove, Kenney and Kendall17 and none evaluated an eye-level strategy. Future research opportunities include evaluating these 3 strategies individually or in combination. An additional gap in the literature is the notable lack of prospective, randomized studies evaluating nudging approaches. Only 1 study in our review was a randomized controlled trial.Reference Daley, Garcia, Inayatullah, Penney and Boyd9 With concurrent ASPs and multiple initiatives at any given time, the potential for confounding variables exists. Future research should aim to evaluate nudging strategies in a randomized and controlled fashion. A limited number of studies evaluated clinical outcomes of nudging, including antimicrobial resistance, C. difficile infection, length of stay, and readmission rates. To improve the robustness of this evidence, clinical outcomes should be evaluated in larger studies that are adequately powered to evaluate these safety metrics.
Nudging approaches are one aspect of many potential behavioral change interventions previously described in the literature.Reference Michie, Richardson and Johnston26 In behavioral-change taxonomy, nudging in the microbiology laboratory commonly fits under the intervention categories of “environmental restructuring” (eg, changing the environment, or in this case the microbiology report, to prompt the prescriber to select the optimal antibiotic), “enablement” (eg, reducing barriers for the prescriber to make the best decision by limiting inappropriate options) or “restriction” (eg, reducing the opportunity for physicians to select a nondesired option).Reference Michie, van Stralen and West27 A broad range of other interventions can be used in place of or as an adjunct to nudging, depending on the specific antimicrobial stewardship challenges and opportunities in a given setting. However, nudging is a relatively low-cost, low-labor-intensity approach to improving antimicrobial stewardship, which is an important consideration for settings with limited funding for ASP human resources.
Nudging in microbiology reporting represents a postanalytic opportunity to influence decision making at the time of prescribing. However, upstream preanalytic approaches may hold significant promise in ensuring appropriate testing and subsequent treatment.Reference Morgan, Malani and Diekema28 A recent study evaluated the impact of changes to computerized physician order entry order sets and the inclusion of a more restrictive reflex urine culturing system.Reference Munigala, Rojek and Wood29 These changes were associated with a 45% reduction in urine cultures ordered. With an increasing interest in diagnostic stewardship and ways to ensure cost-effective resource use and to prevent overutilization of tests, simple nudging approaches will likely have a growing role in all aspects of microbiology.
This scoping review has several limitations. The goal of the review was to ensure breadth rather than depth of evaluation of each research output. Scoping reviews are ideal for exploring heterogeneous topics, but given the heterogeneity in outcomes, scoping reviews typically do not include meta-analyses, which prevented us from drawing conclusions about pooled outcomes among studies. Secondly, the nomenclature describing nudging activities in microbiology laboratory reports is inconsistent; therefore, some studies may have been overlooked. However, our broad search criteria, reference list searching, and grey literature search helped to mitigate the limitations of inconsistent terminology.
The results of this scoping review may be of interest to microbiologists, ASP clinicians, and administrators seeking to identify future opportunities to use or evaluate this promising approach to help guide future research and practice.
In conclusion, a limited number of heterogeneous studies have evaluated the impact of microbiology laboratory nudging, mostly evaluating the impact of altering the default choice or adding framing commentary on the selection of an antibiotic agent. Most of these studies have shown an improvement in antibiotic prescribing behavior. Gaps in research with opportunities for further study include identifying the optimal design of a report to incorporate nudging strategies, performing prospective studies, evaluating the impact of other nudging strategies (eg, including desired agents at eye level or combinations of nudging strategies), and determining the impact of nudging on patient clinical outcomes.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2019.293
Acknowledgments
The authors would like to thank Glyneva Bradley-Ridout, information specialist, for her work on the literature search strategy and article retrieval.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.




