Introduction
Nearly 700,000 people in the United States acquire healthcare-associated infections (HAIs) annually, making HAIs a significant threat to patient, clinician, and public health. Reference Magill, O’Leary and Janelle1–5 Clostridioides difficile infections (CDI) cause about 30,000 U.S. deaths annually, with outbreaks reported in hospitals, nursing homes, and ICUs; spread mainly via the fecal-oral route, its spores persist and require bleach and soap-and-water handwashing for control. Reference Lessa, Mu and Bamberg6–Reference Lakkasani, Chan and Shaaban9 In recent decades, the emergence of new and more virulent C. difficile strains (such as the epidemic B1/NAP1/027 strain) has driven outbreaks and heightened public health efforts, yet key uncertainties in transmission dynamics persist, challenging effective prevention and control. Reference Lawson, Citron, Tyrrell and Finegold10–Reference Nylund, Goudie, Garza, Fairbrother and Cohen14
Mathematical models (infection models) simulate how pathogens spread in healthcare settings—such as understanding how sporadic cases become epidemics—and inform the control of HAIs such as C. difficile. These models which are of different types (Table 1) provide useful insights to support hospital-based and public health decision-making by suggesting potential trends in disease outbreaks and exploring the projected impact of various interventions. Reference Niewiadomska, Jayabalasingham and Seidman15,Reference Starr and Campbell16 While not a substitute for clinical evidence, infection models can complement existing approaches by informing resource allocation, identifying plausible intervention strategies, and simulating “what-if” scenarios in complex healthcare settings. These models can incorporate economic analyses to assess cost-effectiveness, but as simplified versions of real-world settings, they should be interpreted cautiously. However, the accuracy and utility of these infection models, depend heavily on the reliability of the epidemiological parameters used. Reference Lanzas, Jara, Tucker and Curtis17
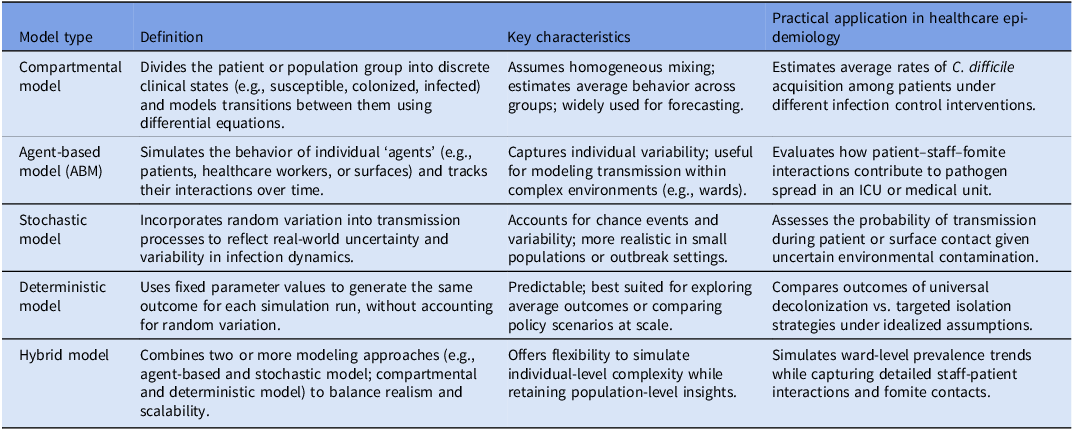
Table 1. Definitions and practical applications of model types used to simulate C. difficile transmission in healthcare settings

Evidence has also shown that fomites, such as environmental surfaces and medical devices, play a critical role in the transmission of HAIs. Reference Gilboa, Houri-Levi and Cohen18–Reference Truong, Schroeder and Gaur23 Several studies, including a systematic review and meta-analysis, have found that patients admitted to wards where a previous patient had C. difficile face an increased risk of acquiring CDI compared to those admitted to a ward with no such history. Reference Faires, Pearl, Berke, Reid-Smith and Weese24–Reference Mitchell, Dancer, Anderson and Dehn30 However, there have been limited studies quantifying high-touch surfaces (HTSs). Reference Link, Kleiner, Mancuso, Dziadkowiec and Halverson-Carpenter31,Reference Wang, Simon, Westblade, Saiman, Furuya and Calfee32 Many earlier studies on the role of environmental surfaces and HTS were qualitative, relying on anecdotal experience, expert knowledge, and assumptions—such as the expectation that objects and surfaces frequently contacted by patients would be the most touched surfaces. Reference Dancer33,Reference Weber, Rutala, Miller, Huslage and Sickbert-Bennett34
This study identifies and summarizes quantitative estimates of key epidemiological parameters to improve the effectiveness of C. difficile infection models. Second, we quantitatively characterize HTS and mutual-touch surfaces in healthcare settings by analyzing contact frequency, contact duration, and temporal variations in contact episodes.
Methods
Literature search strategy and study selection process
We searched four major literature databases—Web of Science, PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Cochrane Database for Systematic Reviews—from the inception of each database until July 8, 2023, for our first objective (C. difficile mathematical modeling parameters) and June 30, 2023, for our second objective (quantifying HTSs). A reference search was also conducted. Additionally, we reviewed gray literature and relevant sources from public health organizations, including the World Health Organization (WHO) website, for both systematic reviews.
For the first systematic review, the search was conducted using Medical Subject Headings (MeSH) and Boolean operators, incorporating three groups of keywords:
-
(1) Mathematical modeling (e.g., “Model*” OR “Mathematical”)
-
(2) C. difficile (e.g., “Clostridioides” OR “Clostridium difficile”)
-
(3) Epidemiological parameters (e.g., “Transmission Coefficient” OR “Recovery Rate”)
For the second systematic review, the search queries included terminologies and synonyms related to major HAI concepts. The keywords and key phrases were categorized into four groups:
-
(1) Fomites, including a list of potential fomites identified from prior literature (e.g., computer, bed rail, supply cart).
-
(2) Contact patterns, including contact frequency, contact duration, and shared-touch patterns (e.g., “high-touch,” “low-touch,” “mutual-touch”).
-
(3) Specific major HAI pathogens, including C. difficile, methicillin-resistant Staphylococcus aureus, among others.
-
(4) Types of healthcare facilities, such as “medical ward” and “surgical ward.”
The complete search strategy for both systematic reviews is provided in Supplementary Document 1.
Inclusion and exclusion criteria for review
For the first review objective, we included mathematical modeling studies that provided evidence on the acquisition and/or transmission of C. difficile and reported at least one infection modeling parameter. We excluded literature reviews, letters to the editor, commentaries, books, and online reports.
For the second review objective, we included studies that met the following criteria: (1) utilized an observational study design, (2) provided quantitative information on at least one of the three study objectives, and (3) were either peer-reviewed journal articles or documents from reputable organizations such as the CDC or WHO. We excluded studies that relied on anecdotal evidence, common knowledge, or expert opinions regarding contact patterns in healthcare settings. Additionally, we excluded qualitative studies that lacked numerical data, non-peer-reviewed documents (except those from reliable sources), books, and summative literature reviews.
Screening process
For both systematic review objectives, we conducted a title and abstract screening to identify studies that met the inclusion criteria. Abstract screening was performed after title screening to allow for a more in-depth evaluation. Studies deemed relevant after abstract screening were retrieved for a full-text review and data extraction. Further details of the screening process are provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagrams (Fig. 1a and 1b).
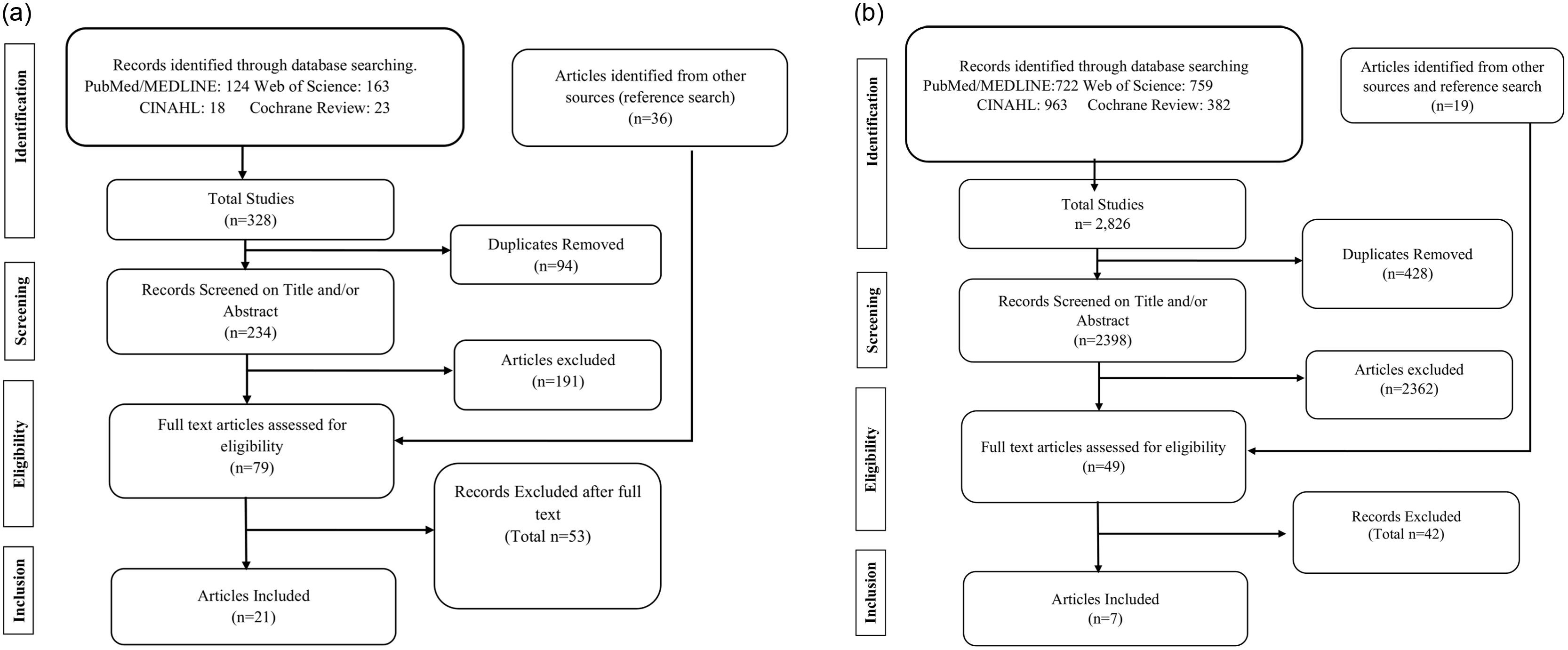
Fig. 1. A PRISMA flow diagram from the screening process to identify eligible studies for systematic review on the epidemiological parameters of C. difficile (Objective 1). b PRISMA flow diagram of the systematic review on the quantitative summarization of high-touch surface studies (Objective 2).
Data extraction and management
We extracted relevant information from the final set of included studies following the screening process. For the first systematic review, the extracted data were categorized into two major groups. The first category was metadata, which included publication details such as title, authors, and year of publication. The second category was model-related data, including the structure of the infection model which refers to how patients, staff and surfaces are represented: compartmental models group individuals into infection disease categories (e.g., susceptible, infected, recovered) during their simulation of pathogen spread, while agent-based models track individual “agents” (such as patients or staff) and their interactions during their simulation process. Another aspect is the dynamics of the model refer to how uncertainty and variability are handled: deterministic models produce the same result for the same inputs, stochastic models incorporate randomness to reflect real-world variation, and hybrid models combine both approaches. This distinction helps clinicians understand both the level of detail and the predictability of model predictions for infection control planning.
For the second systematic review, the extracted information included study details such as author list, study period, study design, study duration, major findings, HAI pathogens, list of fomites and/or surfaces, contact frequency, and contact duration, among other relevant factors. For the overall ranking of HTS, we calculated a cumulative total ranking score by averaging rankings from three or more healthcare settings in which HTS were quantitatively assessed. This was achieved by dividing the cumulative total ranking score of each surface by the number of studies that provided quantitative ranking data. The entire screening process for both systematic reviews was managed using EndNote software, version 20.
Systematic review registration
This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42023408483, in accordance with international standards for conducting and reporting systematic reviews. We followed the guidelines of the Cochrane Collaboration and the PRISMA.
Results
For the first systematic review, we identified 328 initial studies from four major databases that matched our search criteria. The distribution of studies across the four databases was as follows: Web of Science (163), PubMed (124), Cochrane Review (23), and CINAHL (18). After deduplication and full-text screening, 21 studies met the eligibility criteria.
The 21 included C. difficile mathematical modeling studies that met our study protocol eligibility criteria included, based on 16 studies (76.2%) used compartmental modeling approaches that group patients into broad categories such as susceptible or infected group, while 5 studies (23.8%) employed an agent-based modeling approach that simulate individual “agents” such as patients or staff. Additionally, 15 studies (71.4%) used stochastic models that include random variation in their simulation approach to reflect real-world transmission, 3 studies (14.3%) applied deterministic models which produces predictable outcomes during the simulation process, and 3 studies (14.3%) utilized hybrid models that blend predictable structure with random variation in their outcomes. The research articles identified originated from nine countries, with the majority conducted in the United States (40%) and Australia (28%).
For the second systematic review, a total of 2,826 studies were screened after searching four major databases: PubMed (722), CINAHL (963), Web of Science (759), and the Cochrane Library (382). A cross-reference search and additional reputable sources yielded 19 additional studies, which were also screened for inclusion eligibility. After removing 428 duplicates, followed by title and abstract screening and eligibility assessment, 7 studies met the inclusion criteria.
More information can be found in Figure 1a and 1b. Additional details on the search strategy for studies included in this review are available in the Supplementary Document attached.
Basic reproduction number (R₀)
Five infection model studies provided estimates of the basic reproduction number (R₀) which is defined as the average number of secondary infections generated by one infectious case in a fully susceptible population. The basic reproduction number (R₀)—ranged from 0.28, suggesting limited hospital spread, Reference McLure, Clements, Kirk and Glass35 to as high as 2.6, which implies sustained in-hospital transmission. Reference Maghdoori and Moghadas36
It has been hypothesized that C. difficile transmission cannot be sustained in hospitals without continuous importation from the community. Other hypotheses emphasize the role of asymptomatic colonization in C. difficile transmission within hospital settings. Reference Niewiadomska, Jayabalasingham and Seidman15,Reference Lanzas, Jara, Tucker and Curtis17
A study estimated R₀ values to be 1.09 in the community, 1.11 in the general population, and 0.28 in hospital settings. Reference McLure, Clements, Kirk and Glass35 Since R₀ was less than 1 in this hospital, this suggests that sustained hospital-based transmission alone may not account for ongoing CDI cases, supporting the hypothesis that infections may originate from community sources in this case and not solely hospital-acquired. Reference McLure, Clements, Kirk and Glass35 In contrast, another study from our review reported the highest R₀ of 2.6 in a hospital setting, suggesting that C. difficile can continue to propagate within hospitals even in the absence of community importation. Reference Maghdoori and Moghadas36 The other infection modeling studies found the R₀ to be 1.07, Reference Lanzas, Dubberke, Lu, Reske and Gröhn37 0.57, Reference Chamchod and Palittapongarnpim38 0.44, and 0.67. Reference McLure, Clements, Kirk and Glass39
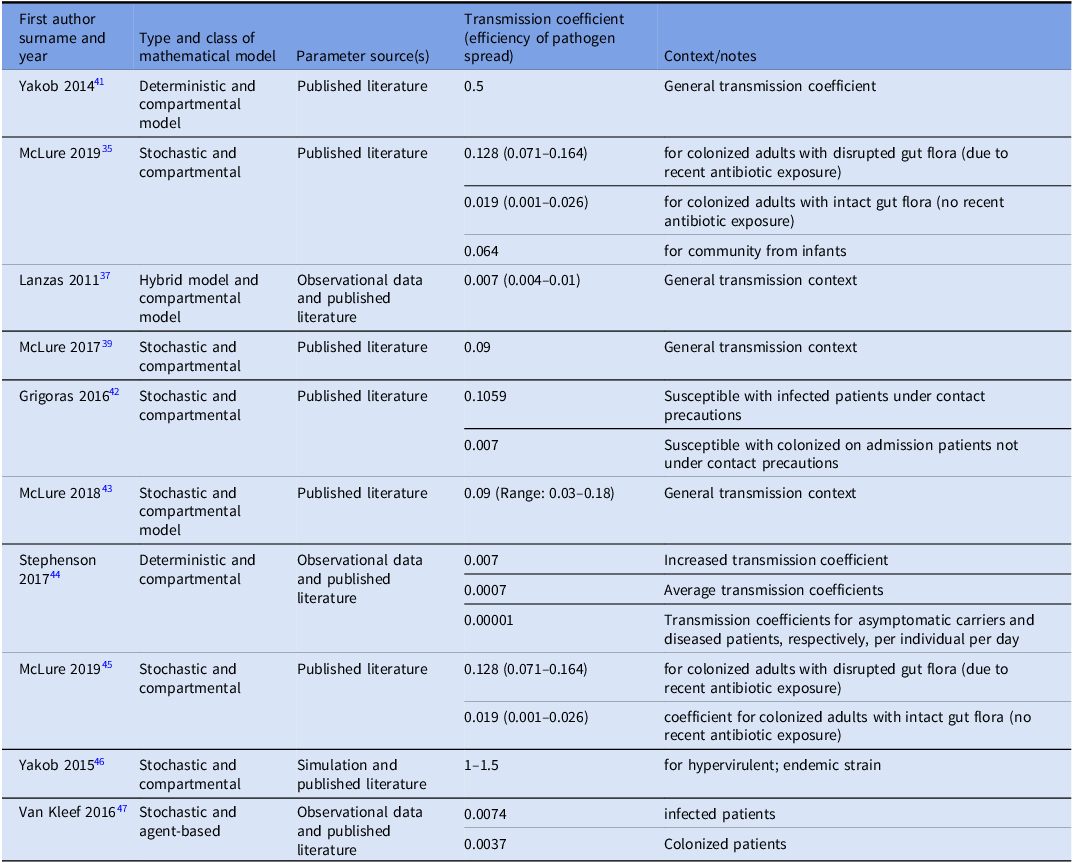
Transmission coefficient
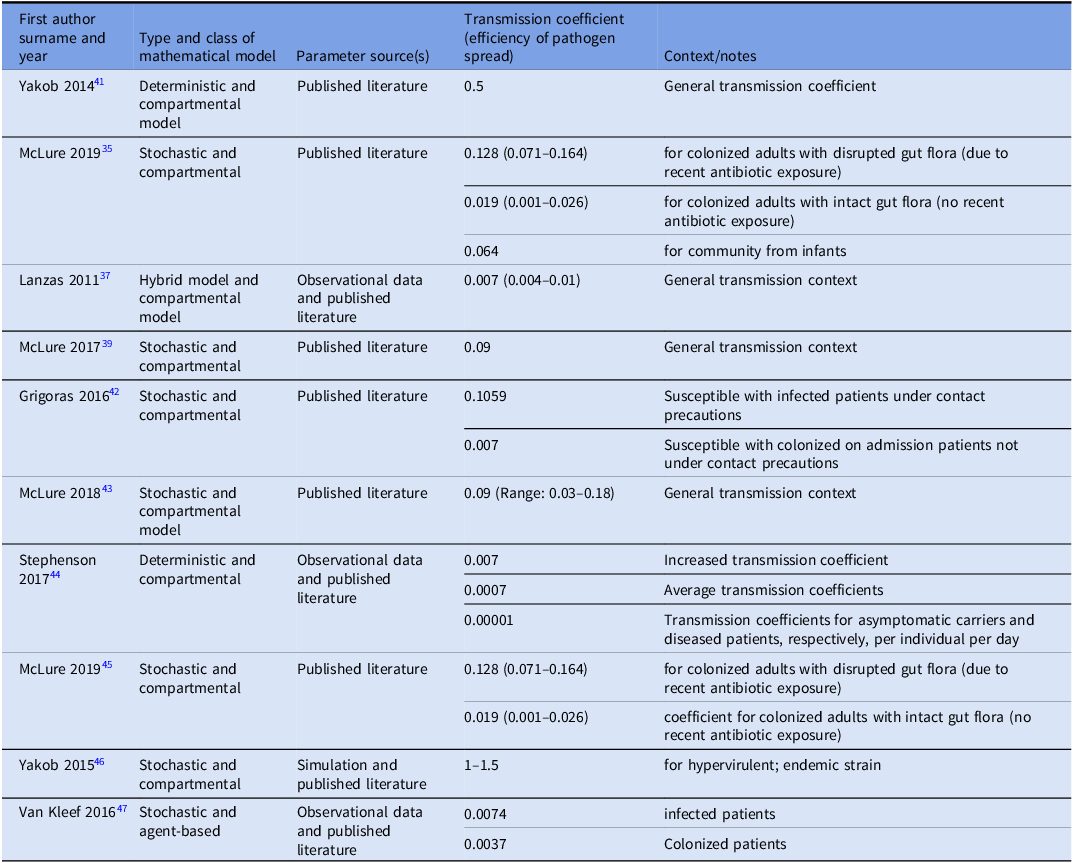
We identified 10 studies that reported transmission coefficients a measure of how efficiently a pathogen spreads; it reflects the likelihood that susceptible individuals become infected through contact with infectious individuals or contaminated environments. Most studies provided estimates for C. difficile transfer between patients, or from both patients and the healthcare environment. Reported transmission coefficient estimates ranged from 0.001 to 0.5, highlighting that the risk of C. difficile spread can vary substantially across hospital settings and infection control practices. Only one study quantified transmission specifically from contaminated fomites (environmental sources). Reference Sulyok, Fox, Ritchie, Lanzas, Lenhart and Day40 See Table 2 for more details.
Table 2. Estimated transmission coefficients for C. difficile spread across different models and contexts
Recovery rate and recurrence rate
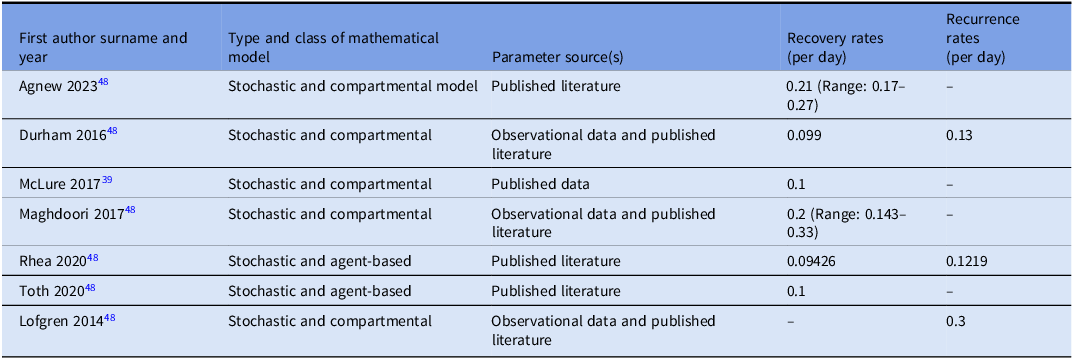
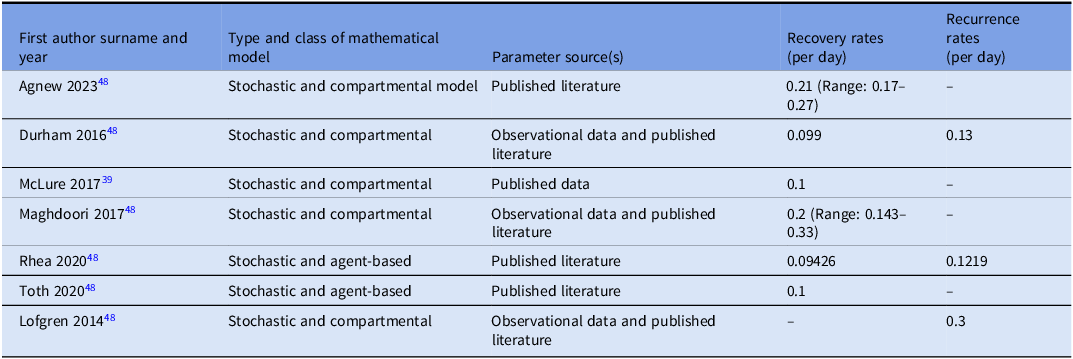
Six studies Reference Agnew, Davies and Viprey48–Reference Toth, Keegan and Samore53 provided information on recovery rates (rate at which infected individuals recover per unit time), which ranged from 0.099 to 0.21 per day, meaning on average 10%–21% of patients with CDI recover each day. The recurrence rate (rate at which a patient experiences another episode of CDI after initial clinical resolution) ranged from 0.13 to 0.3 per day, indicating that nearly one-third of patients who initially improve may relapse within days (Table 3).
Table 3. Estimates of recovery rate or/and the recurrence rate
Hospital discharge rate
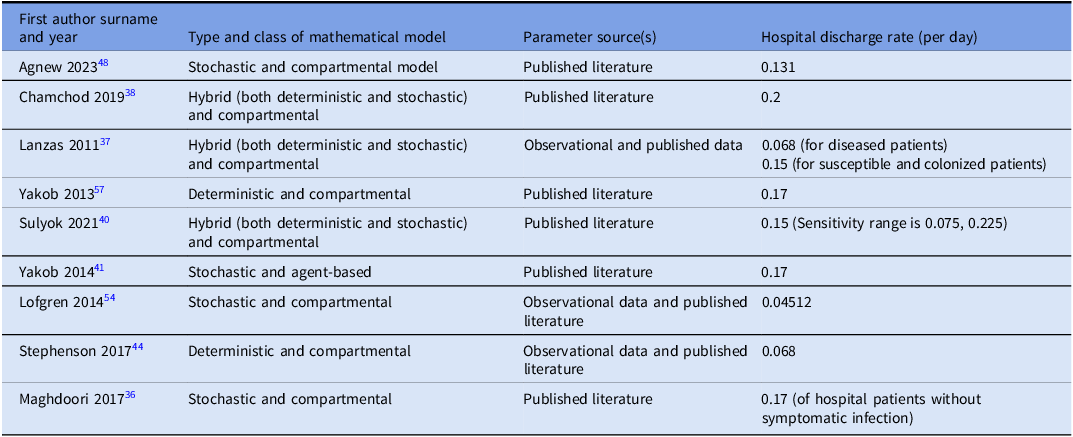
We identified nine studies Reference Chamchod and Palittapongarnpim38,Reference Yakob, Riley, Paterson, Marquess and Clements41,Reference Stephenson, Lanzas, Lenhart and Day44,Reference Lofgren, Moehring, Anderson, Weber and Fefferman54–Reference Maghdoori and Moghadas60 that estimated hospital discharge rates (Table 4). Reported values ranged from 0.04 to 0.2 per day. This broad range reflects variations in patient populations, healthcare settings, and modeling assumptions.
Table 4. Estimates of hospital discharge rates

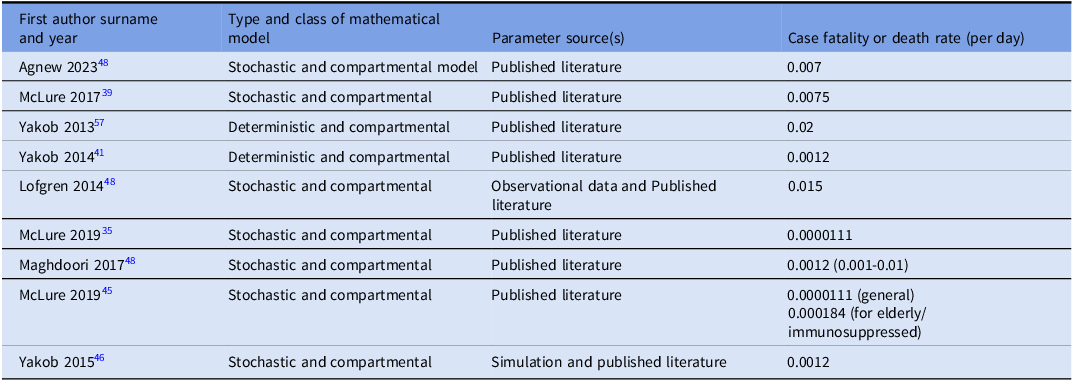
Case fatality rates (CFR)
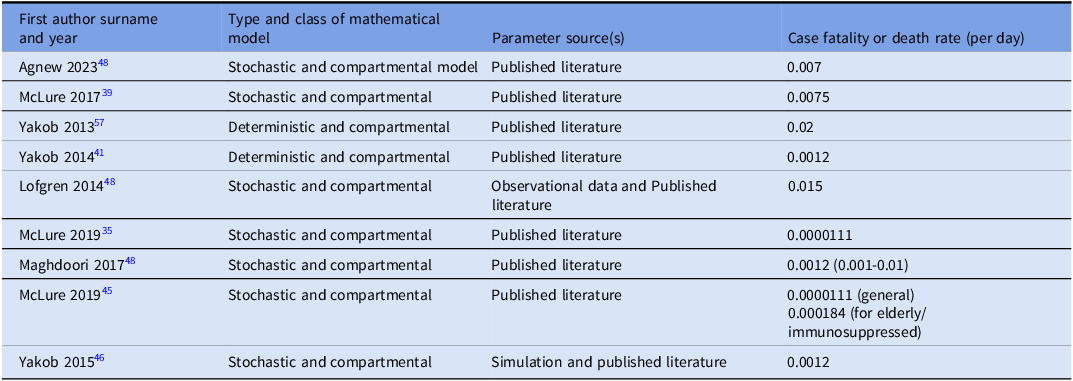
Nine studies reported case fatality rate (CFR) estimates (the proportion of infected individuals who die from the disease) used in their mathematical models. Reported CFR values ranged from 0.0000111 to 0.02 per day (Table 5). CFR values were reported per day to align with model time steps and are not directly comparable to clinical mortality metrics.
Table 5. Estimates of case fatality rates (CFR)
Incubation period
We identified four studies that explicitly estimated the incubation period (time between infection and symptom onset) in their mathematical models. Reference van Kleef, Deeny and Jit47,Reference Rubin, Jones and Leecaster61–Reference Champredon, Shoukat and Moghadas63 These studies reported incubation periods of 4, 5, 6, and 18 days, respectively.
Ranking of high-touch surfaces
We ranked commonly mentioned HTS in the included studies based on their reported ranking in the primary studies across nine healthcare settings. Two studies out of the seven studies that reported on HTS, analyzed HTS in two different healthcare settings, contributing to the total of nine included settings. Reference Wang, Simon, Westblade, Saiman, Furuya and Calfee64,Reference Huslage, Rutala, Sickbert-Bennett and Weber65 The most frequently touched fomites in each hospital setting received a score of 1, the second most frequently touched received a score of 2, and the least frequently touched received the highest numerical score. Overall, the most frequently touched fomite across all nine healthcare settings was the bed rail, with an average ranking score of 2.43. The other high-contact fomites in healthcare settings included bedside tables, supply carts, medication carts, patient notes, the patient’s body, computer keyboards, phones, computer mouses, bed surfaces, intravenous pumps, and vital signs monitors, among others (Fig. 2).

Fig. 2. Ranking of high-touch surfaces across healthcare settings.
Mutual-touch surfaces
We identified several mutually touched fomites based on evidence from the reviewed studies. Two studies provided quantitative data on mutual-touch surfaces.
Wang et al (2021) reported that the most commonly touched mutual surfaces were supply cart drawers, handwashing faucet handles, video translator machines, medication dispensers, supply cart surfaces, thermometers, scales, portable vital signs machines, and light switches, with contact frequencies of 17.1, 10.33, 3.0, 2.5, 2.0, 1.5, 1.0, and 0.4 contacts per hour, respectively. Reference Wang, Simon, Westblade, Saiman, Furuya and Calfee64
Cheng et al (2015) found that the most frequently touched mutual surfaces included the bedside rail, bedside table, patient body, patient file, linen, curtain, bed frame, and locker, with total recorded contact-episodes per hour of 13.6, 12.3, 9.4, 9.3, 6.1, 4.3, and 3.6, respectively. Reference Cheng, Chau and Lee66
Contact duration
None of the reviewed studies quantitatively measured contact duration, although they provided quantitative data on contact frequency for various fomites and surfaces.
Assessment of heterogeneity and temporal variation in contact patterns
There was high heterogeneity among the included studies, as they used different measurement indices to quantify contact structures in healthcare settings. While three studies reported the total number or frequency of touches, Reference Cheng, Chau and Lee66–Reference Jinadatha, Villamaria and Coppin68 two studies used “contacts per hour.” Reference Wang, Simon, Westblade, Saiman, Furuya and Calfee64,Reference Cheng, Chau and Lee66 Huslage et al (2010) measured the “mean number of contacts per interaction,” Link et al (2016) reported the “mean frequency of touch,” and Suwantarat et al (2017) used “interactions per patient per hour” as a contact measurement. Reference Huslage, Rutala, Sickbert-Bennett and Weber65,Reference Link, Kleiner, Mancuso, Dziadkowiec and Halverson-Carpenter69,Reference Suwantarat, Supple, Cadnum, Sankar and Donskey70 Additionally, two studies employed covert observational techniques, while the remaining five studies used direct observations.
Among the reviewed studies, only one study provided evidence of seasonal variations in contact frequency. The authors found that surface and patient contact episodes were more frequent during the summer compared to the winter. This finding was statistically significant (P = 0.002), with the odds of observing patient and/or surface contact activity in the summer being more than twice that of the winter period (CI: 0.269–0.738). Reference Smith, Young, Robertson and Dancer67
Discussion
Our study synthesizes quantitative evidence on essential parameters for CDI models which simulates how infection spreads in healthcare settings, including the basic reproduction number (R₀), incubation period, and recovery rate. Additionally, we provide a quantitative summary of HTSs in healthcare settings. As our systematic review provide evidence that a small number of fomites (surfaces) account for most contact episodes in healthcare settings, our findings suggest that targeted disinfection of frequently and mutually touched surfaces, such as bed rails and supply carts, may reduce environmental transmission and could be prioritized in resource-limited settings.
Our results also indicate a persistent paucity of studies that specifically estimate and/or explicitly report parameters for C. difficile infection models. Notably, different infection model types can produce different results because they make different assumptions. Compartmental models average outcomes across patients, while agent-based models capture variation and uncertainty, sometimes yielding wider ranges for intervention impact. Hence, model outputs should be interpreted cautiously and tailored to their specific setting.
Most of the modeling studies included in this review focused on hospital settings, particularly acute care facilities. Other settings, such as nursing homes and long-term care facilities, have been less frequently investigated. The lack of comprehensive surveillance data is a major limitation in expanding these models beyond acute care hospitals. Reference Asgary, Snead and Wahid71 Clinicians making decisions about infection prevention should note that infection models can help forecast the impact of enhanced cleaning strategies, patient isolation, or antibiotic stewardship interventions.72 Given the complexity of C. difficile transmission across different settings, key epidemiological parameters used in these models must be carefully verified and validated for each context.
Our study has several major strengths: the comprehensive synthesis of quantitative evidence on both C. difficile transmission parameters and the contribution of HTSs across healthcare settings, providing actionable insights for infection prevention planning. Also, we adhered to well-established systematic review methodologies, including guidelines from the Center for Reviews and Dissemination and PRISMA. Furthermore, we provide recommendations for improving mathematical models based on our findings.
One major limitation of current C. difficile modeling studies is the limited number of studies that provide R₀ estimates. When R₀ is estimated to be high (>1), suggesting sustained in-hospital transmission, hospital epidemiologists may prioritize stricter isolation protocols, enhanced cleaning, or environmental surveillance. In contrast, if R₀ is low (<1), indicating community importation, efforts may shift toward improving admission screening, diagnostic testing, or outpatient prevention strategies.
To translate these findings into practice, infection prevention teams should consider conducting environmental audits of high-touch and mutual-touch surfaces, especially in wards with high CDI incidence. Quantifying these contacts could help refine cleaning protocols and prioritize surfaces that pose the highest transmission risk. Our findings provide valuable insights to inform and improve future mathematical modeling studies on C. difficile transmission dynamics. By synthesizing key epidemiological parameters, this study facilitates easier identification and use of summarized quantitative estimates across different model, aiding researchers in developing more accurate models.
To conclude, accurate parameter estimates are critical for developing mathematical models that inform infectious disease control. Our study has summarized key parameters for modeling the acquisition and transmission of C. difficile across various settings and has provided quantitative evidence on human-environment contact patterns, particularly HTSs in healthcare settings. While gaps in evidence remain, modeling studies should expand beyond acute care hospitals. Our findings will strengthen infection control strategies by summarizing key infection modeling parameters to enable healthcare teams to simulate and prioritize the most effective interventions, optimize cleaning protocols, and refine C. difficile transmission models for more targeted CDI prevention.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2025.10302
Financial support
The project described was supported by cooperative agreement (U01CK000677) from the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
Competing interests
The authors declare no competing interests.