Introduction
Antibiotic-resistant bacteria infect 2 million Americans annually, resulting in up to 100,000 deaths and excess healthcare costs exceeding $20 billion.Reference Boucher, Talbot and Bradley1,2 Antibiotic use is a major contributor to antibiotic resistance, Clostridioides difficile infections (CDI), and antibiotic-associated adverse events. Antibiotics are frequently used across all healthcare settings in the United States, although much of this use is unnecessary.Reference Polk, Hohmann, Medvedev and Ibrahim3–Reference Weiner, Fridkin and Aponte-Torres6 In response, antibiotic stewardship programs (ASPs) have sought to coordinate efforts to improve antibiotic prescribing.Reference Tamma and Cosgrove7 Although there has been much progress with antibiotic stewardship (AS) over the past decade, gaps in optimizing the reach and effectiveness of AS remain. We convened a diverse, multidisciplinary group of AS clinicians and researchers to delineate and prioritize these research gaps from a US human health perspective.
We highlight 4 broad categories in which gaps exist (Table 1): (1) a scientifically rigorous evidence base to define optimal antibiotic prescribing practices, which adequately inform AS interventions across a variety of patient populations and settings; (2) effective AS approaches to recognize effective interventions, knowledge of how these interventions can be adapted for implementation both locally and across diverse settings, and an understanding of how interventions can be sustained once implemented; (3) standardized process and outcome metrics; and (4) advanced study designs with appropriate analytic methods, accompanied by infrastructure to support data collection and sharing.
Table 1. High-value targets for antibiotic stewardship (AS) research
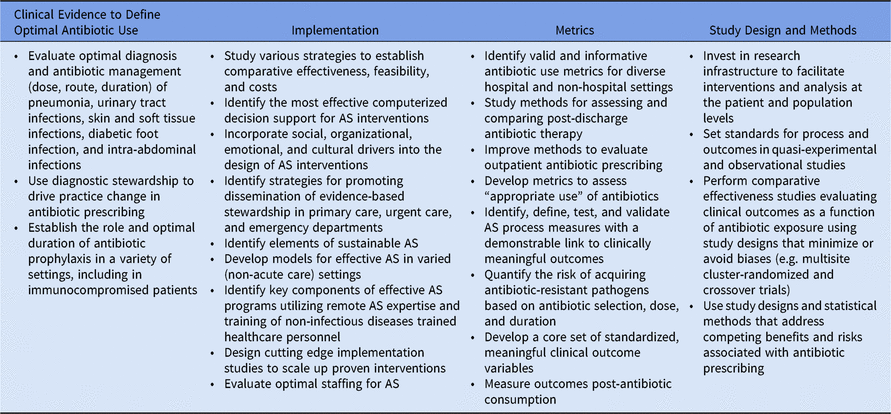
Clinical evidence to define optimal antibiotic use
Antibiotic stewardship is the effort to improve appropriate antibiotic use, at the correct dose, by the proper route of administration, for a sufficient (but not excessive) duration, and only when benefits outweigh potential risksReference Barlam, Cosgrove and Abbo8; however, defining “appropriate antibiotic use” can be challenging without supportive data from well-designed studies.Reference Spivak, Cosgrove and Srinivasan9 Early attempts at assessing appropriateness have used concordance with guidelines to help adjudicate prescribing behavior.Reference Ciccolini, Spoorenberg, Geerlings, Prins and Grundmann10,Reference Dresser, Bell and Steinberg11 Yet this approach is flawed: national guidelines do not always prioritize treatment approaches, frequently exclude clinically relevant populations (eg, immunocompromised patients, older adults, etc), and generally fail to address other aspects of infection management, such as the role of source control.Reference Khan, Khan, Zimmerman, Baddour and Tleyjeh12,Reference Lee and Vielemeyer13 The need for up-to-date, evidence-informed guidance based on the principles of antibiotic stewardship that provides management recommendations informed by relevant patient characteristics and priorities is clear.14 In addition, dosing to optimize pharmacokinetics and pharmacodynamics still needs to be defined for several patient populations frequently excluded from clinical trials (eg, obese patients, patients requiring renal replacement therapy, patients with altered volumes of distribution, etc) or those infected with organisms with elevated antibiotic minimum inhibitory concentrations. Evidence guiding the selection and duration of antibiotic therapy in immunocompromised patients is also largely deficient. Herein, we describe major research gaps in the clinical evidence for infectious disease syndromes that are relevant to AS. Although these can best be evaluated using randomized clinical trials, well-designed cost-effective studies, such as pragmatic trials, adaptive or Bayesian designs, and observational studies using scientifically rigorous approaches to adjust for confounding by indication, can provide valuable data to inform policy and practice.
Diagnostic issues in pneumonia
Several additional issues surrounding pneumonia warrant more investigation. First, although classic systemic symptoms, respiratory physical exam findings, and new or evolving infiltrates on chest imaging make the diagnosis of bacterial pneumonia highly likely, ambiguities with the transcribing of imaging findings and atypical symptoms at presentation may sway clinicians toward prescribing antibiotics when the presentation is more representative of fluid overload, aspiration events, pulmonary infarctions, or viral infections.Reference Wunderink and Waterer15 Although advances have been made in our use of laboratory diagnostic stewardship (ie, preventing the testing and reporting of test results that are not clinically indicated), further research in “imaging” stewardship would likely lead to reductions in the overdiagnosis of bacterial pneumonia and subsequent overtreatment of noninfectious processes.Reference Morgan, Malani and Diekema16 Second, the role of nasopharyngeal and sputum specimen testing, microbiological processing including use of respiratory pathogen panels to guide treatment decisions (including in the absence of pathogens), requires further study.Reference Musher, Montoya and Wanahita17 Finally, the role of biomarkers in both the diagnosis and management of respiratory tract infections needs further exploration.Reference Huang, Yealy and Filbin18,Reference Schuetz, Christ-Crain and Thomann19
Community-acquired pneumonia (CAP)
Consensus guidelines are available for CAP in both children and adults.Reference Mandell, Wunderink and Anzueto20,Reference Bradley, Byington and Shah21 Well-designed studies are needed to determine whether all mild-to-moderate CAP requires universal antibiotic therapy, perhaps assisted by biomarkers such as procalcitonin, and to determine whether macrolide therapy should always supplement β-lactam therapy.Reference Huang, Yealy and Filbin18,Reference Mills, Oehley and Arrol22–Reference Eljaaly, Alshehri and Aljabri25 Randomized trials evaluating the need for routine treatment of CAP with agents active against atypical pathogens have yielded inconsistent results.Reference Garin, Genné and Carballo26,Reference Postma, van Werkhoven and van Elden27 Additionally, antibiotic durations <5 days may be appropriate to treat CAP in certain adults,Reference Avdic, Cushinotto and Hughes28 but data from adequately powered, multicenter, randomized, controlled clinical trials are needed. High-quality data are also needed to define optimal durations for patients with underlying structural lung disease and for frail, elderly adults cared for in the community. No randomized studies have defined optimal treatment durations for CAP in children, and current national guidelines default to 10 days, acknowledging insufficient data to evaluate shorter courses.Reference Bradley, Byington and Shah21
Hospital-acquired pneumonia (HAP)/Ventilator-associated pneumonia (VAP)
A pivotal RCT supported shortened duration of therapy of ~1 week for VAP, although even shorter courses might be sufficient.Reference Chastre, Wolff and Fagon29 Current guidelines for HAP and VAP likely still result in the overuse of broad-spectrum antibiotics because they recommend relatively low thresholds for the empiric use of anti–methicillin-resistant Staphylococcus aureus (MRSA) and antipseudomonal coverage (including the use of combination therapy) while acknowledging that these are based on low- or very low-quality data.Reference Kalil, Metersky and Klompas30 Studies that have valued reducing invasive procedures (eg, bronchoscopy) over targeted antibiotic therapy have reinforced empiric, broad-spectrum antibiotic therapy.Reference Group31 Subpopulations that truly warrant anti-MRSA and/or antipseudomonal coverage and approaches for safe de-escalation need to be better defined, preferably using validated risk scores, decision trees, and/or non–culture-based and rapid diagnostics. Additionally, the role of inhaled antibiotics for HAP/VAP (and other conditions where they are used or could be used, such as bronchiectasis and cystic fibrosis) would benefit from further study.
Asymptomatic bacteriuria and urinary tract infections (UTIs)
Asymptomatic bacteriuria remains one of the most common reasons that antibiotics are inappropriately prescribed for acute- and long-term-care patients. Research focused on the relative sensitivity and specificity of specific UTI symptoms may help to reduce excess antibiotic use for asymptomatic bacteriuria, as would establishing threshold urinalysis parameters that warrant proceeding to culture. One of the most common reasons urine cultures are obtained in older adults is changes in mental status.Reference Nicolle, Gupta and Bradley32–Reference Daniel, Keller, Mozafarihashjin, Pahwa and Soong34 Additional research is needed to better establish how mental status changes (or subtle changes from baseline clinical status) in older adults predict the likelihood of UTIs,Reference Nace, Drinka and Crnich35 and to identify criteria for collecting urine cultures in nonverbal, critically ill patients.Reference Nicolle, Gupta and Bradley32 Also, the benefit of screening for and treatment of asymptomatic bacteriuria in pregnancy remains unknown, despite widespread practice.Reference Smaill and Vazquez36–Reference Moore, Doull and Grad38
The practice of ordering urine diagnostic studies in the absence of relevant signs and symptoms represents an ongoing driver of unnecessary antibiotic prescribing, and interventions are needed to direct testing and the interpretation of test results within the context of a specific patient.Reference Daniel, Keller, Mozafarihashjin, Pahwa and Soong34 Reported strategies include (1) only performing urine cultures if urinalysis results are suggestive of a UTI, (2) not reporting the results of urine cultures with low quantities of bacterial counts, (3) not reporting the results of urine cultures when organisms unlikely to warrant therapy are recovered (eg, Candida spp), and (4) as has been successfully demonstrated in one study, not reporting urine culture results unless the prescriber calls the clinical microbiology laboratory requesting culture results.Reference Gupta, Hooton and Naber39–Reference Epstein, Edwards and Halpin42 Further studies demonstrating the safety and effectiveness of these approaches are necessary, perhaps also investigating the role of biomarkers in this spectrum of illness.
Several randomized studies have evaluated the selection, route, and duration of antibiotic therapy for both cystitis and pyelonephritis, with most of these studies limited to otherwise healthy women.Reference Talan, Stamm and Hooton43,Reference Sandberg, Skoog and Hermansson44 However, comparative effectiveness trials comparing 7 or fewer days of both fluoroquinolone and nonfluoroquinolone antibiotics for pyelonephritis are needed. Additional studies are also needed to inform optimal durations of therapy in male patients and those with comorbidities that impact urinary tract functionality or UTI risk (eg, multiple sclerosis, diabetes mellitus, and renal transplantation).
Skin and soft-tissue infections (SSTIs)
Several randomized trials have informed effective treatment regimens for SSTIs, with treatment durations generally ranging from 5 to 10 days, but the optimal treatment duration remains unclear. In one study, 7 days of cephalexin alone was shown to be equivalent to 7 days of cephalexin plus trimethoprim-sulfamethoxazole for nonpurulent cellulitis.Reference Moran, Krishnadasan and Mower45 Further studies evaluating shorter antibiotic courses for uncomplicated cellulitis would be informative, as one single-center study suggested that 5 days of therapy may be sufficient for uncomplicated cellulitis.Reference Hepburn, Dooley, Skidmore, Ellis, Starnes and Hasewinkle46 In addition, 2 randomized trials evaluated treatment strategies for uncomplicated skin abscesses.Reference Talan, Mower and Krishnadasan47,Reference Daum, Miller and Immergluck48 Both studies found that 7–10 days of antibiotic therapy in addition to incision and drainage was associated with improved clinical cure and relapse rates. Further studies are needed to evaluate antibiotic courses <7–10 days and to assess whether antibiotic therapy is still warranted for drained cutaneous abscesses when S. aureus is not identified. Additionally, studies including immunocompromised patients or patients with prosthetic material are necessary.
Diabetic foot infections (DFIs)
Diabetic foot infections represent a condition with considerable morbidity and mortality in affected patients.Reference Uçkay, Berli, Sendi and Lipsky49,Reference Lipsky, Berendt and Cornia50 Despite the importance of this condition, it remains poorly studied. In addition to the importance of examining approaches to prevent DFIs, studies addressing optimal choice, route, and duration of both empiric and pathogen-directed therapy are needed, including clarifying the role of clinical, radiographic, and microbiologic tests.
Intra-abdominal infections (IAIs)
Although national guidelines have outlined treatment recommendations for both community-acquired and hospital-associated IAIs,Reference Solomkin, Mazuski and Bradley51 several areas warrant further investigation. For example, treatment regimens for mild-to-moderate community-associated IAIs (eg, ciprofloxacin plus metronidazole and ceftriaxone plus metronidazole) do not provide enterococcal coverage, and interventional studies that explore whether targeted therapy is needed when these organisms are identified are lacking. Similarly, limited evidence indicates the benefit of adding antifungal agents to treatment regimens when Candida spp are recovered from patients with mild-to-moderate community-acquired IAIs. In addition, the relationship between obtaining cultures routinely (and providing targeted therapy) and clinical effectiveness remains uncertain.
Although RCT data have been used to define durations of therapy for IAI when source control has been achieved,Reference Sawyer, Claridge and Nathens52 additional evidence is needed to guide the appropriate therapy when source control is suboptimal (ie, drainage has not occurred, abscesses remain, or fistulae are present). Additional research is also warranted to identify optimal treatment duration for infants with necrotizing enterocolitis and patients with neutropenic enterocolitis. Finally, considering the increasing prevalence of antibiotic-resistant Escherichia coli, future studies should investigate the effectiveness of commonly prescribed oral step-down regimens for IAIs.
Bacterial prophylaxis
Antibiotic prophylaxis has been shown to prevent infections.Reference Enzler, Berbari and Osmon53 For some infectious syndromes, however, data to guide effective and judicious antibiotic prophylaxis are lacking. Further work is needed to define the role of prophylactic antibiotic regimens for recurrent UTIs. Additionally, observational data suggest patients at high risk for recurrent C. difficile infections may benefit from secondary prophylaxis while on systemic antibiotic therapy.Reference Carignan, Poulin and Martin54,Reference Van Hise, Bryant, Hennessey, Crannage, Khoury and Manian55 Randomized controlled trials are needed to confirm these findings.
Preoperative antibiotic prophylaxis decreases the risk of surgical site infectionsReference Bratzler, Dellinger and Olsen56; however, several unanswered questions remain regarding perioperative antibiotic use that require further research, such as the value of weight-based dosing for specific antibiotics, of intra-operative redosing of antibiotics, and of any postoperative dosing.Reference Bratzler, Dellinger and Olsen56 Prolonged postprocedural antibiotic prophylaxis is commonly used when prosthetic material, drains, and high-risk invasive devices are placed (eg, extracorporeal membrane oxygenation), but there is little evidence to support these practices and more data evaluating benefits and harms are needed. Finally, in 2007, revised guidelines from the American Heart Association narrowed the indications for antibiotic prophylaxis to prevent infective endocarditis in patients with pre-existing valvular disease.Reference Wilson, Taubert and Gewitz57 Other jurisdictions, such as the United Kingdom, have narrowed the indications for infective endocarditis prevention dramatically further, but not without controversy.Reference Chambers, Shanson, Venn and Pepper58–Reference Cahill, Harrison and Jewell60 A large trial is needed to definitively address when and whether prophylaxis for infective endocarditis is beneficial.
Anti-infective prophylaxis is commonly prescribed for immunocompromised patients, but for many conditions the optimal approach to prophylaxis is unclear.Reference Tomblyn, Chiller and Einsele61,Reference Taplitz, Kennedy and Bow62 Future studies should clarify which patients with allogenic hematopoietic stem cell transplants and hematologic malignancies benefit the most from antibacterial and antifungal prophylaxis, including prophylaxis against Aspergillus spp and other molds. Patients who receive chemotherapy and have an anticipated period of neutropenia of at least 7 days benefit from fluoroquinolone prophylaxis, but it remains unknown whether antibiotic prophylaxis is still beneficial if the neutropenia persists for an extended period (eg, several months) and whether the benefit outweighs the harm associated with antimicrobial resistance.
Finally, the risk of developing antibiotic resistance and C. difficile colonization/infection while receiving antibiotic prophylaxis is poorly understood. Providing clinicians with better data on the risks of antibiotic prophylaxis could inform shared decision making between patients and providers about whether prophylaxis is warranted.
Antibiotic stewardship implementation
Although numerous evidence-based AS interventions have been described, particularly in acute-care hospitals, the uptake of these interventions has been incomplete, and effectiveness is often variable.Reference Pogorzelska-Maziarz, Herzig, Larson, Furuya, Perencevich and Stone63–Reference Davey, Marwick and Scott65 First, AS implementation research requires a mixed methods approach with attention to behavioral science, human factors, and systems engineering.Reference Rzewuska, Charani and Clarkson66 Existing stewardship interventions need refinement to improve their impact and efficiency across different healthcare settings and disciplines while new strategies to change prescriber behavior are identified and evaluated. Second, research is needed to define the factors that shape the sustainability of AS interventions once implemented. Third, there is a need for evidence that demonstrates the optimal configuration of personnel needed to lead stewardship across healthcare settings with variable resources. Much of healthcare is now provided outside of hospital settings. Stewardship interventions that are effective in nonhospital settings are poorly defined. Research priorities regarding interventions for nonhospital settings are further highlighted in Box 1.
Box 1. Antibiotic stewardship research gaps for interventions in non-hospital settings
1. Ambulatory Care: While there has been a number of high-quality studies exploring the usefulness of antibiotic stewardship interventions pertaining to acute respiratory tract infections, there are a number of critical questions left unanswered.Reference Meeker, Linder and Fox67,Reference Meeker, Knight and Friedberg68
a. What can outpatient antibiotic stewardship programs do to address appropriate prescribing for other community-acquired infections, such as urinary tract infection and skin and soft tissue infections?
b. How can patient expectations be met while antibiotic stewardship principles are being upheld?Reference Arnold and Straus69
c. How can public health campaigns influence antibiotic-prescribing behaviors over the long-term?
2. Emergency Departments: Antibiotic stewardship research involving the emergency department has generally been of poor quality.Reference Losier, Ramsey, Wilby and Black70 We identify several gaps including:
a. What is the role of multi-disciplinary teams in antibiotic stewardship, and which healthcare professional should be involved in the stewardship intervention?
b. Are stewardship strategies that have proven effective in primary care also prove effective in the ED?
3. Long-Term Care: Antibiotic stewardship research in long-term care facilities is limited, and has had mixed results. Reference Feldstein, Sloane and Feltner71,Reference Katz, Gurses, Tamma, Cosgrove, Miller and Jump72 Although the influences of antibiotic prescribing in long-term care is viewed to be multifactorial, prescriber factors appear to be dominant. Reference Daneman, Gruneir and Bronskill73 Research priorities (considering both short-stay and long-stay residents) include:
a. How can hospitals and post-acute care nursing facilities collaborate toward antibiotic stewardship goals?
b. What are the most effective stewardship strategies for long-term care?
c. When cultures from non-sterile body sites are positive, how can clinicians more effectively distinguish infection from colonization, particularly in mentally impaired patients who cannot report symptoms?
d. How can the nursing staff be incorporated into stewardship efforts and what tools can be developed to improve communication between the nursing staff and antibiotic prescribers?
e. How can antimicrobial stewardship be integrated within Quality Assurance Performance Improvement activities
Antibiotic stewardship interventions
Current guidelines on the implementation of ASPs for acute-care hospitals include descriptions of numerous strategies to optimize antibiotic use.Reference Barlam, Cosgrove and Abbo8 These strategies can be categorized as either restrictive (eg, prior authorization) or persuasive (ie, do not force a certain behavior, but rather “nudge” it such as with prospective audit with feedback [PAF]) based upon the mechanism used to change prescriber behavior.Reference Davey, Marwick and Scott65 The evidence supporting audit and feedback in acute-care settings is robust.Reference Ivers, Jamtvedt and Flottorp74 A systematic review of hospital-based AS interventions demonstrates that both restrictive and persuasive interventions increase adherence to prescribing guidelines but that adding a persuasive component to restrictive interventions enhances their effect.Reference Davey, Marwick and Scott65
Although increasing evidence indicates that AS strategies are effective, understanding of which combinations of strategies are most effective is lacking, especially accounting for the impact on patient outcomes, resistance, and cost.Reference Naylor, Zhu, Hulscher, Holmes, Ahmad and Robotham75 Prospective audit with feedback improves antibiotic prescribing, but it is labor intensive, typically involving a review of cases by dedicated practitioners.Reference Cosgrove, Seo and Bolon76 Research to increase the efficiency of this strategy is needed to allow broader implementation. Peer comparison of antibiotic use via personalized e-mail reports have facilitated improved prescribing in the outpatient setting.Reference Meeker, Linder and Fox77,Reference Hallsworth, Chadborn and Sallis78 Combining peer comparison with PAF in inpatient settings may lead to greater improvements in antibiotic use than either method alone. Delivering feedback via in-person rounding with prescribing clinicians (eg, “handshake stewardship”) has shown promise, but further study in facilities with a range of resources available is needed before it can be widely implemented.Reference Hurst, Child, Pearce, Palmer, Todd and Parker79,Reference Hurst, Child and Parker80
Providing clinicians with information at the point of prescription via computerized decision support (CDS) has the potential to improve antibiotic prescribing.Reference Curtis, Al Bahar and Marriott81 For example, an organization’s electronic medical record (EMR) could facilitate antibiotic time-outs, dose optimization, and de-escalation.Reference Rawson, Moore and Hernandez82 Data available in the EMR could also be leveraged to develop validated risk scores for risk of antibiotic-resistant organisms and to aid in empiric antibiotic selection and subsequent de-escalation. Development of CDS-based strategies that ignore the sociotechnical context frequently lead prescribers to work around or ignore them.Reference Cresswell, Mozaffar, Shah and Sheikh83 High-quality comparative studies are needed to identify the most effective CDS-based AS interventions, with accompanying implementation strategies to optimize prescriber engagement.Reference Curtis, Al Bahar and Marriott81,Reference Baysari, Lehnbom, Li, Hargreaves, Day and Westbrook84,Reference Bond, Chubaty and Adhikari85 In addition, the impact of implementing CDS in healthcare settings that lack trained AS professionals (eg, postacute care and small rural hospitals) needs to be evaluated.
Emotional factors, such as clinicians’ fear of the “worst-case scenario,” their fear of possible negative patient satisfaction scores if they withhold antibiotic therapy, or their desire to avoid conflict with a demanding patient, have been demonstrated to drive antibiotic overuse.Reference Teixeira Rodrigues, Roque, Falcão, Figueiras and Herdeiro86–Reference Broom, Kirby, Gibson, Post and Broom88 Antibiotic prescribing in inpatient settings is sensitive to the social dynamics within groups of clinicians, including hierarchy, professional power, and shared accountability.Reference Charani, Castro-Sanchez and Sevdalis89,Reference Papoutsi, Mattick, Pearson, Brennan, Briscoe and Wong90 Research is needed to assess the feasibility and effectiveness of stewardship interventions that target social and emotional dynamics. Stewardship interventions in nonhospital settings that target clinician-patient and clinician-caregiver communication behaviors, particularly during care transitions, are needed.Reference Mangione-Smith, McGlynn, Elliott, McDonald, Franz and Kravitz91–Reference Mangione-Smith, Elliott, Stivers, McDonald and Heritage93 Intervention trials evaluating communication techniques that both reduce antibiotic prescribing and maintain patient satisfaction are critical for frontline prescribers, and early studies have demonstrated promise.Reference Légaré, Labrecque, Cauchon, Castel, Turcotte and Grimshaw94 Although promising examples of investigators adapting and reporting behavioral economics principles in stewardship research are available, future work must describe the behavioral components of interventions with specificity and precision.Reference Meeker, Linder and Fox77,Reference Michie, Richardson and Johnston95–Reference Hulscher, Grol and van der Meer97
Sustainability of stewardship interventions
Studies evaluating the benefit of inpatient stewardship interventions have demonstrated significant short-term reductions in antibiotic prescribing and hospital length of stay without increasing mortality or otherwise adversely impacting patient outcomes.Reference Smith, Gerber and Hersh98–Reference Honda, Ohmagari, Tokuda, Mattar and Warren101 Other studies have provided some limited evidence of short-term improvements in both long-term care and ambulatory settings.Reference Drekonja, Filice and Greer102,Reference Feldstein, Sloane and Feltner103 The evidence supporting the utility of ASPs in the emergency department is less clear.Reference Losier, Ramsey, Wilby and Black104 Analysis has typically been restricted to the period immediately following the intervention, with limited evaluation of the sustained impact during a maintenance period, such as a re-evaluation 3 years or more after the intervention. Changing the behavior of prescribers does not happen spontaneously, and it may be difficult to sustain improvements in prescribing over time as clinicians fall back into old patterns, especially as the intensity of the original intervention has decreased.Reference Gerber, Prasad and Fiks105–Reference Linder, Meeker and Fox107
Personnel for effective stewardship programs
Infectious diseases (ID) physicians and pharmacists have largely taken a leadership role in stewardship activities to date. There is a research gap, however, in how to conduct stewardship within healthcare facilities that have limited or no access to ID expertise. Some remote ID specialists have been able to externally facilitate local stewardship activities in hospital settings with limited resources.Reference Bishop, Kong, Schulz, Thursky and Buising108,Reference Stenehjem, Hersh and Buckel109 Future studies should describe how this approach influences both antibiotic prescribing and related clinical outcomes. Additionally, the role of remote expertise in support of stewardship implementation in postacute care facilities can be immensely beneficial and efficient but has not been adequately evaluated.Reference Kullar, Yang, Grein and Murthy110,Reference Mody, Washer and Flanders111
Future research should also identify effective ways to train non-ID healthcare personnel to support local stewardship activities.Reference Honda, Ohmagari, Tokuda, Mattar and Warren101,Reference Dik, Vemer and Friedrich112 Nurses have largely been absent from the AS landscape, even though engagement with nurses may prove a promising method of improving stewardship capacity in a variety of settings.Reference Olans, Olans and DeMaria113 More research needs to be conducted to determine how best to engage bedside nurses in stewardship activities and the specific roles they could play.Reference Jeffs, Law and Zahradnik114 Furthermore, since antibiotics are prescribed by medical and surgical specialists and subspecialists, strategies to engage these professions in AS need to be developed and evaluated.
As facilities increasingly develop their own stewardship programs, there is a need to better define how stewardship programs should be effectively resourced and managed at the organizational level.Reference Pulcini, Morel and Tacconelli115–Reference Doernberg, Abbo and Burdette117 Studies should clarify the amount and type of staffing (full-time equivalents [FTE]) a facility should devote to stewardship activities, accounting for the size of the facility, the complexity of the patient population, the utilization of antibiotics, and the availability of other relevant resources (eg, informatics) that correlate with improved outcomes before saturation is observed.Reference Echevarria, Groppi, Kelly, Morreale, Neuhauser and Roselle118 Furthermore, studies quantifying the human resource needs of different stewardship strategies and their relative impact can help programs, administrators, and regulators make evidence-based decisions in program development and regulatory requirements.
Antibiotic stewardship metrics
Currently, there is no globally accepted metric to assess the success of an ASP.Reference Ng, Ching and Chan119–Reference Aldeyab, McElnay and Scott121 Instead, ASPs rely on available data and resources to determine the impact of AS interventions. This lack of consistency limits generalizability and limits data aggregation for systematic reviews and meta-analyses of multiple studies. The need for valid and reliable metrics to assess the clinical impact of AS efforts is critical to advancing the science of this discipline.
Antibiotic use and process measures
Although antibiotic utilization alone does not adequately quantify the multifaceted goals of ASPs, it is the most commonly tracked process metric. Work to quantify and compare antibiotic use is in its early stages for acute-care hospitals in the United States. As more hospitals participate in the National Healthcare Safety Network (NHSN) Antimicrobial Use (AU) Option, comparisons to national estimates should become more valuable; however, the current methods for such comparisons have yet to be validated, and efforts to optimize metrics are warranted. Developing and applying standardized approaches to epidemiology and antibiotic use reports is necessary.Reference Tacconelli, Cataldo and Paul122 Research should aim to define relevant patient- and facility-level risk adjustment variables to allow for meaningful benchmarking of antibiotic use.Reference van Santen, Edwards and Webb123,Reference Yu, Moisan and Tartof124 This work will be essential before stewards can widely adopt the CDC’s standardized antibiotic administration ratio (SAAR) metric for active use in program assessments.Reference Fridkin and Srinivasan125
Epidemiologic investigations into antibiotic use among postacute care and long-term care settings are needed, both to better define areas of opportunity and to better understand antibiotic use trends. Studies that help refine preprescribing processes to reduce inappropriate investigations and test interventions that target postprescribing decision making, such as de-escalation, will be important to changing practice. Defining and validating definitions for antibiotic use such as “targeted,” “de-escalated,” “appropriate,” and “adequate” therapy are needed.Reference Spivak, Cosgrove and Srinivasan9,Reference Dresser, Bell and Steinberg11 Future multisite studies should evaluate the effects of stewardship interventions on facility-level and patient-level outcomes including healthcare costs, rates of C. difficile infections, and antibiotic resistance. In outpatient settings, the optimal metric for antibiotic use remains unclear. Experts have proposed using an antibiotic-prescribing rate (eg, antibiotic prescriptions divided by predefined patient-visit type).Reference Sanchez, Fleming-Dutra, Roberts and Hicks126,Reference Dobson, Klepser and Pogue127 Additional study is needed to further refine outpatient prescribing metrics so they are directly actionable; are not prone to variation due to differences in billing practices; and can better direct antibiotic surveillance in primary care, emergency departments, urgent care clinics, retail clinics, and virtual visits.
Novel process measures also require standard definitions, validation, assessments of feasibility, and testing for their effect on clinical outcomes.Reference Akpan, Ahmad, Shebl and Ashiru-Oredope128 For example, de-escalation is a core principle of AS but is a general concept, has many interpretations, and lacks a universal definition and method of measurement. Once defined, robust studies can evaluate the impact of de-escalation in the setting of absent or negative cultures to hopefully further promote this practice as both achievable and safe. In addition, hospital-based ASPs infrequently measure antibiotics prescribed (and their duration) at the time of hospital discharge, a large proportion of which are inappropriate.Reference Yogo, Haas, Knepper, Burman, Mehler and Jenkins129,Reference Scarpato, Timko and Cluzet130 Further study is needed to capture these data to develop interventions that improve antibiotic prescribing upon hospital discharge.
Clinical outcome measures
Evaluation of the effectiveness of ASPs needs to move from process to outcome measures.Reference Davey, Marwick and Scott65,Reference McGowan131,Reference Dodds Ashley, Kaye, DePestel and Hermsen132 The stated goals of ASPs are to reduce antibiotic resistance, adverse drug effects, and secondary unintended consequences (eg, CDI). As such, future research needs to focus on the development of objective, well-defined, and standardized outcome measures. Making these fully accessible via the EMR is an additional research imperative.Reference Jones, Haroldsen and Madaras-Kelly133
Significant variability exists in the clinical outcomes assessed in AS studies. Clinical outcomes can include clinical cure/failure, mortality (all cause, infection-related, antibiotic resistance-related), length of stay, hospital readmission rates, antibiotic resistance, incident CDI rates in both the hospital and the community, and antibiotic-related adverse events. Outcome measures, however, are problematic for a variety of reasons: (1) they are influenced by non-ASP interventions, (2) they may be dependent on nonmodifiable factors such as patient case mix and local epidemiology, (3) they are rare events requiring large sample sizes, and (4) they may require significant time after an intervention before an effect is observed. Several groups have proposed outcome measures to assess ASP impact on clinical outcomes in the acute-care setting, but no consensus on specific measures or definitions has been reached.Reference Morris, Brener and Dresser120,Reference Moehring, Anderson and Cochran134 Developing a core set of standardized clinical outcome variables for which stewardship studies can be appropriately designed and powered will drive research to identify the most effective AS interventions in diverse healthcare settings.
It is also critical to measure outcomes associated with AS interventions that may extend beyond the encounter when antibiotics were prescribed. Prior stewardship interventions have indicated that there is no short-term impact on mortality, but adequate evaluation of post-discharge events, including acquisition of resistant pathogens, requires longer follow-up and may not have been captured.Reference Smith, Gerber and Hersh98,Reference Stenehjem, Hersh and Buckel109,Reference Matthaiou, Ntani, Kontogiorgi, Poulakou, Armaganidis and Dimopoulos135 With clear outcome definitions that can be tracked longitudinally after a stewardship intervention, studies could evaluate the impact stewardship interventions have on unintended or avoidable ED or clinic visits, readmissions to the hospital, and the impact on intestinal bacterial diversity (including incident colonization/infection with multidrug-resistant organisms), in addition to mortality.Reference Honda, Ohmagari, Tokuda, Mattar and Warren101,Reference Feldstein, Sloane and Feltner103
Antibiotic stewardship study design and methods
Researchers face multiple challenges in performing high-impact research due to the complex, multilevel nature of AS practice. Many AS interventions are targeted at the provider or system level and not at the patient level. This disconnect in the level of intervention and level of outcome assessment causes complexity in evaluating effects of interventions due to correlation resulting from clustering. In addition, system-, hospital-, clinic-, and provider-level interventions that change healthcare delivery processes make blinding the allocation of the intervention impossible. Finally, studies of impact on rarer clinical outcomes such as antibiotic resistance, require larger populations and multiple practice locations. Therefore, robust assessments of AS interventions require more financial resources and investments in research infrastructure.
Study design and methods tailored to evaluate AS interventions should address several aims. First, investigations must improve the quality of small or single-center quasi-experimental studies by standardizing AS-focused process metrics and reporting important patient-focused outcomes, as discussed above, in addition to process and implementation metrics. Existing meta-analyses of stewardship interventions have generally found large degrees of heterogeneity, mostly due to the variability of methods employed as the collective “intervention” and the variety of outcome metrics utilized.Reference Davey, Marwick and Scott65,Reference Honda, Ohmagari, Tokuda, Mattar and Warren101,Reference Schuts, Hulscher and Mouton136,Reference Baur, Gladstone and Burkert137 Second, methods to identify and avoid major sources of selection, information, and confounding biases in quasi-experimental and observational studies should be better defined with an AS focus.Reference Lederer, Bell and Branson138 Third, evaluation of new initiatives in the context of established, ongoing AS activities will need to be carefully interpreted to understand the incremental changes observed with new activities.
Comparative effectiveness studies evaluating clinical outcomes as a function of antibiotic exposure are less prone to bias when patients are randomized to treatment assignment. For large-scale studies, multisite cluster randomization, crossover, time-series analysis, or stepped-wedge designs could potentially avoid biases in pragmatic trials of system- or practice-level interventions; however, such studies require defined methods for power calculation, estimates of intracluster correlations, and handling of time-related effects (eg, seasonality). Study design and statistical methods should aim to address the need to improve sample size efficiency, account for correlation commonly present in AS studies, and weigh competing risks, using novel approaches.Reference Evans, Rubin and Follmann139
Conclusion
Antibiotic resistance is a growing problem, often driven by unnecessary and inappropriate antibiotic use, and AS is necessary to optimize antibiotic use through evidence-based interventions. Research in AS is entering an exciting era as we move away from demonstrating the need for AS and toward research with a focus on improving patient outcomes. We have identified 4 research priorities: (1) development of evidence supporting best clinical practices in a variety of settings and patient populations (ie, what to do); (2) assessment of optimal approaches to implement AS practices in diverse settings, with a focus on behavioral change, sustainability, and personnel (ie, how best to do it); (3) development of standardized, valid and reliable process and outcome metrics to support AS efforts, supported by information technology infrastructure and analytics (ie, how to measure what you are doing); and (4) approaches to advanced study design with appropriate analytic methods (ie, how to determine effective methods to continually improve stewardship practices). Although this report does not outline all gaps in the evidence for AS, it highlights critical research needs at the vanguard for supporting high-quality patient care and public health.
Acknowledgments
We would like to thank Kristy Weinshel for her substantial support in the preparation of this manuscript.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



