Management Implications
The potential costs of managing escapes from cultivated plantings that become invasive are often undervalued in decisions on whether to introduce a potential invader. Before planned introductions, such as the case of biofuel crop cultivation, more accurate estimates of the costs of managing potential escaped populations will more effectively inform decisions on whether and where to introduce and the amount of effort that must be spent on containment or eradication. Miscanthus species, including Miscanthus × giganteus (giant Miscanthus hybrid) and Miscanthus sinensis (eulaliagrass), are prime targets for bioenergy production because of their efficient biomass production; however, both species have the potential to become invasive if individuals escape cultivation. We quantified the costs associated with eradicating introduced populations of M. sinensis and M. × giganteus at three old field and three floodplain forest sites in central Illinois. We found no survival of Miscanthus individuals of either species within the floodplain forest site after the first herbicide application. In contrast, at two of the old field sites, a small percentage (less than 5%) of Miscanthus plants proved persistent, surviving repeated annual herbicide applications and even surviving attempts to excavate the plants to a depth of 40 cm. The time, effort, and costs associated with eradicating Miscanthus populations within the old fields were considerable, ranging from $390 to $3,316 per site (or $1.3 to $11 m−2), compared with only $85 to $547 (or $0.92 to $1.82 m−2) required to eradicate populations within the floodplain forests. Estimating the costs of eradicating feral populations of a potentially invasive cultivated plant enables more empirically informed decisions on the potential costs and benefits that must be considered before large-scale cultivation. Additionally, the total costs of eradication can inform more effective decision making as to when to shift from a goal of eradication to one of containment.
Introduction
The costs associated with managing or not managing invasive species are substantial (Millennium Ecosystem Assessment 2005). Throughout North America, the estimated economic costs of invasive species have totaled more than US$26 billion yr−1 since 2010 (Crystal-Ornelas et al. Reference Crystal-Ornelas, Hudgins, Cuthbert, Haubrock, Fantle-Lepczyk, Angulo, Kramer, Ballesteros-Mejia, Leroy, Leung, López-López, Diagne and Courchamp2021), which include the direct costs of managing invasive species and the indirect costs associated with the loss of ecosystem services via disruptions to the invaded community (Panetta Reference Panetta2009; Regan et al. Reference Regan, McCarthy, Baxter, Dane Panetta and Possingham2006). When allocating resources toward invasive species management, the direct costs (costs associated with invasive species control) must be balanced against the indirect costs (the potential spread and associated impacts an invasive species can cause to the invaded habitat). Accurate estimates of the costs of managing feral plant populations are required to effectively inform decisions on whether and where to introduce and the amount of effort that must be spent on containment and eradication.
Cultivated perennial grasses, such as those grown for bioenergy or ornamental purposes, have high potential to escape cultivation and invade surrounding habitats (Lambertini Reference Lambertini2019; Matlaga and Davis Reference Matlaga and Davis2013). In fact, the ideal traits of a bioenergy and ornamental grasses substantially overlap with the traits that confer invasive potential, including a wide range of tolerance to soil and climate conditions and pest and disease resistance (Barney and DiTomaso Reference Barney and DiTomaso2008; Raghu et al. Reference Raghu, Anderson, Daehler, Davis, Wiedenmann, Simberloff and Mack2006). However, large-statured perennial grasses, such as Miscanthus spp., comprise a functional group of effective colonizers and invaders (Lambertini Reference Lambertini2019). While there have been considerable efforts to quantify the potential invasiveness of biofuel candidate crops (Barney Reference Barney2014; Matlaga and Davis Reference Matlaga and Davis2013; Quinn et al. Reference Quinn, Matlaga, Stewart and Davis2011; West et al. Reference West, Matlaga and Davis2014b), to our knowledge, we are currently lacking estimates of the potential monetary costs associated with their management if they invade natural areas.
Miscanthus species, including the giant Miscanthus hybrid (Miscanthus × giganteus J.M. Greef & Deuter ex Hodkinson & Renvoize) and eulaliagrass (Miscanthus sinensis Andersson), are prime targets for bioenergy production because of their efficient biomass production. Miscanthus sinensis was initially introduced in North America as an ornamental grass in the late 1800s, and since then, escapes have formed naturalized populations, at times in near-monotypic stands, in a diverse array of habitats throughout the eastern United States (Quinn et al. Reference Quinn, Allen and Stewart2010). However, whether feral Miscanthus populations can reduce species richness and diversity of the native plant community is unclear (Hager et al. Reference Hager, Rupert, Quinn and Newman2015; West et al. Reference West, Matlaga, Muthukrishnan, Spyreas, Jordan, Forester and Davis2017).
To limit invasiveness, Miscanthus × giganteus, a cross between M. sinensis and Amur silvergrass [Miscanthus sacchariflorus (Maxim.) Franch.], was promoted as a biofuel candidate because of its sexual sterility (Linde-Laursen Reference Linde-Laursen1993) and limited clonal expansion (Jørgensen Reference Jorgensen2011; Matlaga et al. Reference Matlaga, Quinn, Davis and Stewart2012). Recent work, however, casts doubt as to whether sterile cultivars of M. × giganteus have eliminated the invasion threat. For example, multiple studies have highlighted the risk of rhizome propagules dispersing and forming feral populations (Quinn et al. Reference Quinn, Matlaga, Stewart and Davis2011; West et al. Reference West, Matlaga and Davis2014a), which has also been observed in other rhizome-spreading sexually sterile grasses (Khudamrongsawat et al. Reference Khudamrongsawat, Tayyar and Holt2004). Additionally, molecular studies revealed widespread misclassification of commercially available Miscanthus accessions, including misidentification occurring at the species and genotype levels (Oladeinde Reference Oladeinde2012; Perrier et al. Reference Perrier, Hardion, Rozan, Staentzel and Combroux2019). Fertile M. × giganteus genotypes mistaken as infertile have a high potential to invade surrounding habitat. Additionally, rare recombination events can break down triploid sterility, thereby generating fertile gametes in “sterile” populations (Ramsey and Schemske Reference Ramsey and Schemske1998). Spatially explicit demographic models predict that only a small fraction of M. × giganteus seeds need to be fertile to produce a rapidly spreading feral population (Matlaga and Davis Reference Matlaga and Davis2013; Muthukrishnan et al. Reference Muthukrishnan, West, Davis, Jordan and Forester2015).
Currently, we lack data quantifying the potential costs of managing escapes from cultivated plants, as well as the cost of eradication. Estimating the cost of eradication can help effectively inform decisions as to when to shift from a goal of eradication to one of containment.
We conducted a study to evaluate both the feasibility and the time and effort required to eradicate populations of M. × giganteus and M. sinensis in experimental plantings within floodplain forest and old fields. Our primary objectives included: (1) track survivorship (as a metric of efficacy) of M. sinensis and M. × giganteus over the eradication process; (2) quantify costs associated with eradication; and (3) predict the potential costs associated with eradicating existing invasive M. sinensis populations in the United States. A secondary objective was to evaluate the factors (including Miscanthus species and starting plant tiller number) contributing to efficacy of control tactics and eradication costs.
Materials and Methods
Study Sites and Species
Miscanthus species and the design of experimental plantings are described in West et al. (Reference West, Matlaga, Muthukrishnan, Spyreas, Jordan, Forester and Davis2017). In summary, in April 2010, greenhouse-grown plugs of M. sinensis and M. × giganteus were planted in three old field and three floodplain forest sites (Supplementary Figure 1). We chose old field and floodplain habitats for our study because these are dominant habitats in central Illinois and commonly found adjacent to agricultural production areas and are therefore likely to be receptor habitats for Miscanthus escapes from production fields via movement by water (floodplains) or wind and machinery (old fields) (West et al. Reference West, Matlaga and Davis2014a, Reference West, Matlaga, Muthukrishnan, Spyreas, Jordan, Forester and Davis2017). Forested floodplains have remained uncultivated, whereas old field sites are often located on farmland too unproductive to remain in cultivation. The old field sites included Phillips (PH; 40.1346°N, 88.150°W) and Trelease Prairies (TR; 40.127°N, 88.098°W), and the Vermillion River Observatory (VRO; 40.064°N, 87.562°W). Floodplain forest sites included Nanney (NAN; 39.883°N, 88.177°W) and Richter Tracts (RIC; 40.08°N, 87.812°W) and Homer Lake (HL; 40.061°N, 87.980°W). Miscanthus plants were introduced at each site in 2010, and there was a starting population of 116 plugs per species spread over four replicates each, with subplots varying in Miscanthus density. Plugs that did not produce green material were removed and replanted in 2011 and 2012. From 2012 until eradication efforts began in 2014, the only management of the Miscanthus populations occurred at the old field sites, which were mowed annually in the spring to a height of 7.5 to 10 cm to inhibit woody encroachment. Floodplain forests were unmanaged and subject to frequent and occasionally prolonged flooding (West et al. Reference West, Matlaga, Muthukrishnan, Spyreas, Jordan, Forester and Davis2017).
Monitoring Miscanthus Eradication Efforts and Survival
When eradication efforts began in summer 2014, we applied an initial herbicide application of a mixture of both clethodim (Select Max®, Valent, Walnut Creek, CA; 0.27 kg ai ha−1) and glyphosate (WeatherMax®, Bayer Crop Science, St Louis, MO; 1.5 kg ai ha−1) across all Miscanthus plots at each site (Supplementary Table 1). The exception was one of the floodplain forest sites, RIC, because by August 2014 there were no longer living Miscanthus plants apparent aboveground, and therefore herbicide was not applied. In 2014, we also applied an additional application of glyphosate (4.2 kg ai ha−1) to the old field sites in November. As herbicide impacts were similar regardless of whether the mixture or single chemical were applied, all subsequent herbicide applications (starting in September 2014, and beyond) consisted of spot-spraying glyphosate (1.25% v/v) alone. Plants and resprouting tillers declined over time with repeated herbicide application, but some persisted despite successive years of herbicide application. In May and October 2018, the remaining intractable Miscanthus plants were excavated by removing all belowground plant material to a minimum depth of 40 cm, after which all detected rhizomes were excavated from the soil until the rhizome end was fully removed. This was expected to effectively eliminate resprout potential from already depleted rootstocks.
To quantify Miscanthus survival in response to eradication efforts, we recorded the number of plants and tillers per plant for both Miscanthus species at all sites. We considered a Miscanthus species “locally eradicated” after 3 yr of finding no living plants of that species at that site, after which there were no longer any additional costs associated with eradication efforts. For populations in which local eradication was never achieved, the University of Illinois Committee on Natural Areas continued the monitoring and treatment of remaining Miscanthus populations after October 2019.
Estimating Costs of Eradication
A full accounting of cost estimates associated with eradication of both Miscanthus species at each site includes both on-site costs and travel costs. On-site costs comprise the total costs associated with Miscanthus eradication incurred at the site and would not vary based on the distance a land manager must travel to a particular site. Therefore, on-site costs are directly applicable to any site infested with Miscanthus. On-site costs include: (1) labor costs associated with the monitoring and treatment of Miscanthus and (2) cost of herbicides and equipment used in eradicating Miscanthus (Supplementary Table 2). In contrast, the eradication costs associated with travel (including labor costs for travel and costs of vehicle ownership and operation) vary depending on the distance a land manager travels to a site (methodology and estimates of travel costs are found in Supplementary Tables 4 and 5 and Supplementary Figure 3).
To determine the hourly wage for labor devoted to eradication of Miscanthus populations, in 2020, we informally surveyed seven private companies that control invasive plants throughout the eastern and midwestern United States for their average estimates of the hourly wage charged per person for all personnel involved in invasive plant management. The hourly wage reported for invasive plant management varied greatly, ranging from $34 to $107 h−1, with a mean hourly rate of $54 h−1. Because labor costs were by far the largest expense incurred in eradication efforts, we used the minimum, maximum, and mean reported hourly wage to estimate labor costs associated with eradication activities.
Starting in August 2014, we recorded all personnel hours (number of people multiplied by number of hours) spent on both the monitoring and control of Miscanthus populations within each site (Supplementary Table 2). It is important to note that because we were eradicating planned introductions, the exact location of each plant was known preinitiation of Miscanthus eradication efforts. Therefore, we had no initial effort to demarcate the gross infested area, which likely led to conservative estimates for the cost of control and eradication compared with unplanned feral populations in natural settings.
To determine the number of personnel hours spent on eradicating each Miscanthus species at each site, we multiplied the number of personnel hours per site (devoted to either spraying herbicides or excavating plants) by the proportion of living tillers of each species at each site. We assumed that each visit to a site to treat Miscanthus populations required one half hour of preparation, and therefore we added one personnel half hour of preparation onto each site visit. The number of personnel hours spent monitoring each Miscanthus species per site was assumed to be equal to the total number of personnel hours spent surveying for surviving Miscanthus plants at each site, because both Miscanthus species were equally distributed over the entire site area.
For each year, we calculated the sum of all personnel hours spent on eradication efforts and monitoring Miscanthus populations at each site. We then multiplied the minimum, maximum, and mean hourly wage estimates (estimated via the survey of invasive species management companies) by all personnel hours to determine the average and potential range of on-site costs associated with labor. Finally, we calculated the cumulative costs of labor spent on eradication efforts per site, as well as herbicides and equipment, across the 5-yr study duration.
The amount of both clethodim and glyphosate applied per site were quantified in 2014. However, after 2014, the remaining Miscanthus plants were spot sprayed with a 1.25% solution of glyphosate, and the exact amount of herbicide used at each site was no longer quantified. Therefore, to estimate the amount of herbicide used after 2014, we estimated the area sprayed as the estimated area of each Miscanthus plant based on the number of tillers. To do this, we used data collected in 2014 for the number of tillers and area per plant from Miscanthus plants at all sites before initial herbicide application. Area per plant was modeled as a function of tillers by fitting a power curve using the drm function within the drc package in R (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015), and the final model was used to estimate the plant area based on the number of tillers of surviving plants in years after 2014 via the following equation:
where a is a constant specific to each Miscanthus species, X is the number of tillers of plant i, and b is the shape parameter of the curve (Onofri 2019). Finally, the amount of herbicide applied in 2014 and the estimated amount of glyphosate applied in subsequent years were multiplied by the annual estimated price of glyphosate and clethodim (University of Nebraska–Lincoln Extension 2014–2019; Supplementary Table 2).
The costs of equipment used in eradication included a backpack sprayer and a shovel. These were both considered to be upfront costs incurred in 2014 at the beginning of the eradication efforts (see Supplementary Table 2 for price estimates). Equipment costs were only applied to the sites in which that equipment was used. For example, no Miscanthus plants were present at the RIC site by the start of eradication efforts, therefore the sprayer costs were applied to all other sites except RIC.
We then calculated the total on-site costs associated with each Miscanthus population by summing the cumulative labor costs spent on monitoring and eradicating Miscanthus populations with the total costs of herbicide and equipment used at each site. Finally, to relativize the estimated costs by area, for each site, we divided the total on-site costs by the infested area (300 m2).
Factors Associated with Eradication Efficacy and Cost
To evaluate whether eradication costs varied by Miscanthus species and habitat, we used the gls function within the nlme package for linear mixed-effects models in R (Pinheiro et al. Reference Pinheiro, Bates, DebRoy and Sarkar2020). Data were checked for assumptions, including normality, and because all data were heteroscedastic, we used the varIdent function within nlme to group variances by habitat. Fixed effects in the model were Miscanthus species (M. sinensis and M. × giganteus) and habitat (floodplain and old field), as well as their interaction.
To evaluate whether the probability of surviving glyphosate and clethodim applications differed between Miscanthus infestations based on species or plant size (measured by tiller number), we applied logit regression using the glmer function in the lme4 package in R, with both Miscanthus species and tiller number as fixed effects, and site as a random effect.
Predicting the Cost of Eradicating Miscanthus sinensis Populations in EDDMapS
We utilized our estimated on-site costs of M. sinensis eradication to predict the potential costs associated with eradicating existing invasive M. sinensis populations in the United States (there are no confirmed populations of M. × giganteus on EDDMapS). To do this, we queried EDDMapS (eddmaps.org, accessed October 17, 2019) for all positively confirmed cases of M. sinensis. Of the 1,347 positively confirmed M. sinensis cases, however, only 212 cases reported a nonzero estimate of infested area. Using these 212 cases of reported M. sinensis–infested areas, we generated a frequency distribution and assumed that the infested area of all 1,347 reported Miscanthus infestations followed this distribution. We then randomly sampled this distribution 1,347 times to estimate an infested area for each of the positively confirmed M. sinensis infestations on EDDMapS.
To project the on-site costs of eradicating M. sinensis populations reported in EDDMapS, we ran 10,000 simulations in which we: (1) randomly selected values within our estimated cost range ($ m−2, ranging from the minimum reported hourly wage at the site with the lowest cost, to the highest reported hourly wage at the site with the greatest costs) and (2) multiplied the randomly selected on-site cost against our randomly selected infested area ($ m−2 × m2) to get a vector of estimated costs for the 1,347 random Miscanthus sites, and then summed that vector to get an estimated cost of eradication for all Miscanthus sites. We then calculated the minimum, maximum, mean, and median of projected costs from the 10,000 simulation runs.
Results and Discussion
Miscanthus Survival in Response to Eradication Efforts
The feasibility of eradicating Miscanthus populations differed between the old field and floodplain forest sites (Figure 1) but did not differ between the Miscanthus species. We found no survival of Miscanthus individuals of either species within the floodplain forest sites after the first herbicide application. In fact, many of the Miscanthus individuals had died even before the start of eradication efforts, likely the result of shading and competition from neighboring plants or persistent flooding (West et al. Reference West, Matlaga, Muthukrishnan, Spyreas, Jordan, Forester and Davis2017). Over the course of the 5 yr of eradication efforts, only 2.5 to 3.8 h per site (or approximately 0.01 h m−2) were required to monitor and spray Miscanthus plants at the floodplain forests sites (Table 1).
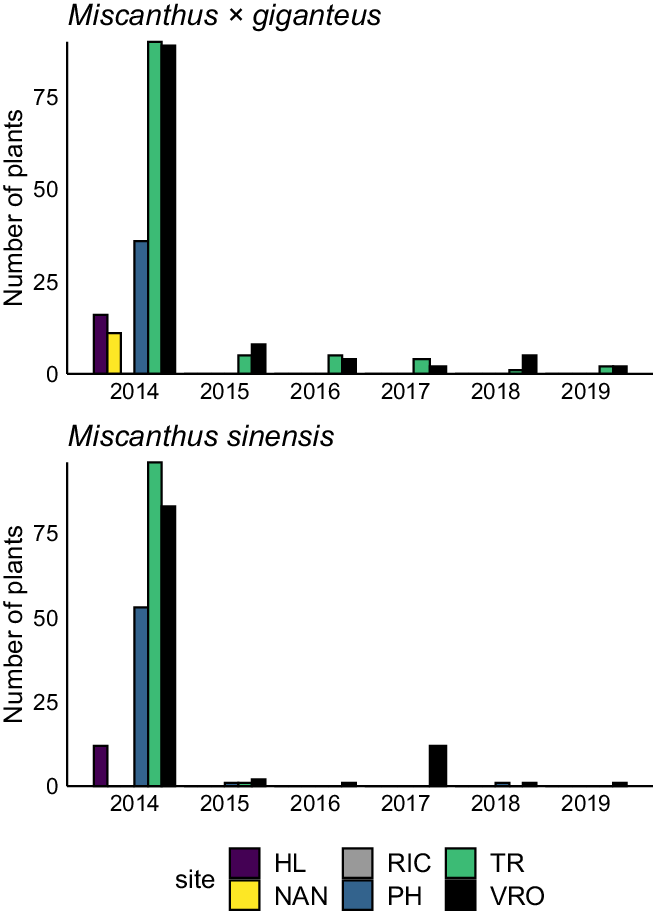
Figure 1. The number of Miscanthus × giganteus and Miscanthus sinensis plants present at sites in 2014 before eradication efforts and in subsequent years after eradication efforts commenced. Sites included three within floodplain forests: Homer Lake (HL), Nanney (NAN), and Richter (RIC); and three within old fields: Phillips (PH) and Trelease (TR) Prairies and the Vermillion River Observatory (VRO).
Table 1. Number of personnel hours spent monitoring and treating existing Miscanthus plants at each Miscanthus infestation site in either the floodplain forests (FF) or old fields (OF) in Illinois.
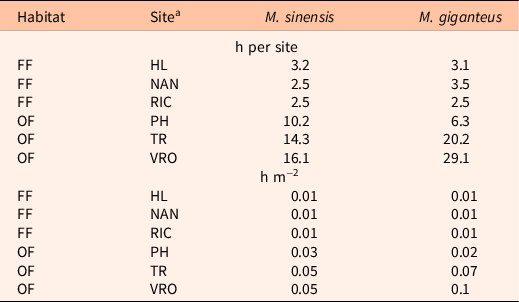
a Sites included: Homer Lake (HL), Nanney (NAN), and Richter (RIC); Phillips (PH) and Trelease (TR) Prairies; and the Vermillion River Observatory (VRO).
In contrast to the floodplain forest sites, a small percentage of the plants sprayed with herbicide persisted within the old field sites. For example, at the TR site, 4.4% and 1.0% of the M. × giganteus and M. sinensis plants, respectively survived the first herbicide eradication attempts; while at the VRO site, 8.9% and 2.4% of the M. × giganteus and M. sinensis plants survived, respectively (Figure 1). Interestingly, while we had expected that larger, more established plants would be more likely to survive initial herbicide applications (in 2014), we found no relationship between the size of the plant (measured as number of tillers) and the probability of survival of the first-year herbicide applications (P > 0.05; Supplementary Figure 2). However, we did find that within the TR and VRO sites, M. × giganteus plants were more likely to survive herbicide applications within the first year compared with M. sinensis (P = 0.004; 6.7% chance of M. × giganteus survival of herbicide applications in old field sites compared with a 1.7% survival of M. sinensis plants; Figure 1). This is likely because M. × giganteus has a larger and deeper network of rhizomes (Enloe and Loewenstein Reference Enloe, Loewenstein, Quinn, Matlaga and Barney2015), and the systemic herbicide glyphosate may not have been able to distribute throughout the rhizome network of all budding root tissue in high enough concentrations. Anderson et al. (Reference Anderson, Voigt, Bollero and Hager2011) showed that M. × giganteus shoot numbers were reduced by as much as 94% over two seasons with two glyphosate applications per year, but shoot numbers were only reduced by approximately 86% within one glyphosate application per year. Therefore, it is possible we were not aggressive enough in the number of glyphosate applications per year.
The number of hours spent on eradication efforts between old field sites varied from 11 h at the PH site, to 36 h at the VRO site. The TR and VRO sites required repeated herbicide applications (Supplementary Tables 2 and 3) over 3 yr, after which the Miscanthus populations at these sites were reduced to only 1 M. × giganteus at TR and 2 M. × giganteus and 12 M. sinensis plants at VRO by 2017. Two of the M. sinensis plants at the VRO site were new escapes that dispersed outside the experimental plots.
After 2017, the decision was made to excavate the remaining plants at both the TR and VR sites, requiring 3 and 16 on-site personnel hours, respectively. Despite the extensive efforts to excavate Miscanthus plants in May 2018 (approximately 35% of personnel hours at the VRO site were spent on excavating the plants) up to eight M. × giganteus plants were found at the VRO site in October 2018. After excavation at the TR site, Miscanthus plants were not observed for an entire year and were considered to be eradicated, only to emerge the following year. It is likely that small rhizome fragments large enough to form new plants remained in the soil after excavation efforts. The high level of disturbance that occurred through repeated herbicide applications and Miscanthus plant excavation may have facilitated reinvasions. By 2021, there were still Miscanthus plants present at both the VRO and TR sites.
On-Site Eradication Costs
Total on-site costs associated with Miscanthus eradication efforts varied by habitat (F = 16.1, P = 0.004), but did not vary between the Miscanthus species (Figure 2; Supplementary Table 3). Total on-site costs (assuming the mean hourly wage of $54 h−1) ranged from $135 to $361 ($0.45 to $1.20 m−2 infested area) in the floodplain forest sites and from $514 to $1,771 ($1.70 to $5.90 m−2 infested area) in the old field sites. Despite the large variability in time and effort required to control or eradicate varying species of invasive plants, our estimated eradication costs are comparable (when considering costs of the gross infested area) to the costs of eradicating Rubus species in Santa Cruz, Galapagos, in Ecuador (Buddenhagen Reference Buddenhagen2006) and invasive Spartina in the San Francisco Bay, California (Jardine and Sanchirico Reference Jardine and Sanchirico2018).
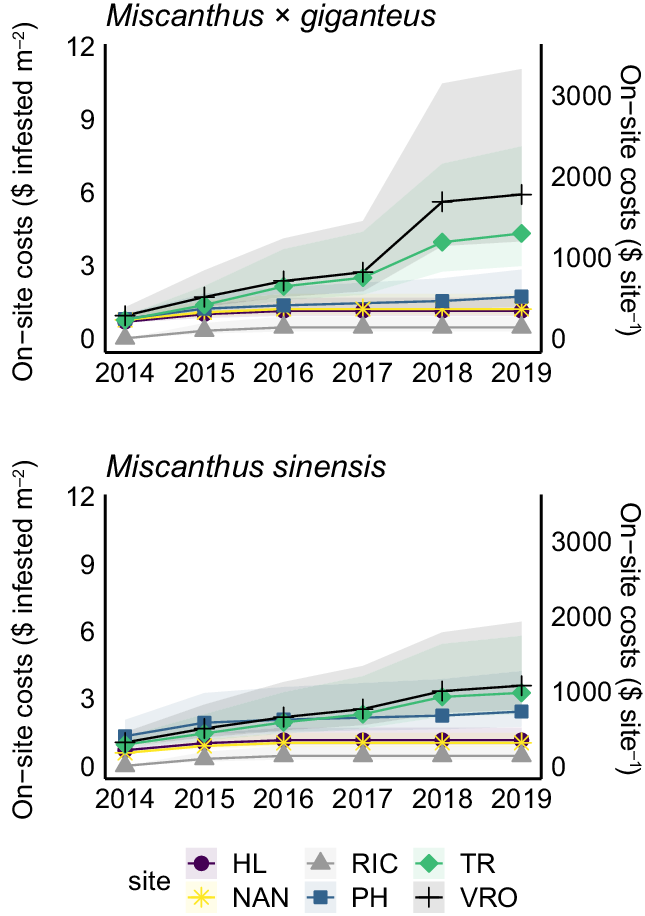
Figure 2. Cumulative on-site costs associated with efforts to monitor and eradicate populations of Miscanthus × giganteus and Miscanthus sinensis plants. Sites included three within floodplain forests: Homer Lake (HL), Nanney (NAN), and Richter (RIC) Tracts; and three within old fields: Phillips (PH) and Trelease (TR) Prairies and the Vermillion River Observatory (VRO). Shaded region represents the area between the maximum and minimum cost estimates based on the maximum and minimum hourly wage estimates.
Labor comprised the largest share of on-site eradication costs, ranging from $135 to $188 ($0.45 to $0.63 m−2 infested area) in the floodplain forest sites and from $337 to $1,556 ($1.12 to $5.19 m−2 infested area) in the old field sites (assuming the mean hourly wage of $54 h−1). Labor costs are more variable among the old field sites, because existing Miscanthus plants were excavated (a much more labor-intensive endeavor) in 2018 in two of the three sites after multiple years of herbicide applications being unsuccessful. Despite the time-intensive nature of digging up invasive plants, this approach is consistent with eradication efforts reported in other studies in which plants were excavated (Scott et al. Reference Scott, Batchelor and Webber2019).
In addition to labor, other on-site costs included the purchasing of equipment and herbicide used for eradication (see Supplementary Table 2 for details). Equipment costs ranged from $0 to $200, the bulk of which is attributed to the backpack sprayer ($170) and was incurred in the first year of the study. Comparatively, estimated herbicide costs were minimal ($7 or less per site). No herbicide was sprayed at the RIC site, because the Miscanthus populations had all died at that site before the initiation of eradication efforts; therefore the RIC site incurred $0 in equipment or herbicide costs.
Estimating Eradication Costs for Miscanthus Populations in the Eastern United States
We found 1,347 positively confirmed cases of M. sinensis on the EDDMapS website. We assumed that the cost to eradicate these populations fell within our estimated on-site range between $0.28 m−2 (assuming the minimum reported hourly wage of $34 h−1) to $6.42 m−2 (assuming the maximum reported hourly wage of $107 h−1). Applying our cost estimate ranges for the M. sinensis populations in Illinois, we predict that the potential costs that would be incurred to eradicate these Miscanthus populations would range from $10 to $37 million, with a median predicted cost of $22 million.
We attempted to estimate the full costs incurred to eradicate feral Miscanthus populations (including travel costs; see Supplementary Tables 4 and 5). It is important to note that our eradication cost estimates are likely conservative, and therefore, underestimate the true cost of Miscanthus eradication. Most importantly, our Miscanthus invasion sites were preplanned, so we initially knew the locations of each infestation and initial efforts to demarcate the gross infested area was zero. In contrast, Miscanthus populations confirmed on EDDMapS are not as well demarcated; therefore each confirmed Miscanthus location on EDDMapS would require land managers to engage in wider search efforts to confirm the extent to which the population had spread. Other studies have found that more than 50% (Buddenhagen Reference Buddenhagen2006) and as high as 75% (Tye Reference Tye2007) of personnel hours devoted to eradicating an invasive plant consists of search efforts surrounding known infested areas.
Second, the Miscanthus populations targeted within our study were young, having only established four years before the start of eradication efforts. Likely, these younger plants were easier to eradicate compared with denser and more mature Miscanthus stands that have a more established network of rhizomes (Matlaga et al. Reference Matlaga, Quinn, Davis and Stewart2012), thereby diluting the concentration of the systemic herbicide glyphosate as it spreads through the root system (Ziska et al. Reference Ziska, Faulkner and Lydon2004). Eradication of invasive species becomes increasingly difficult and cost prohibitive as they establish and expand geographically. Additionally, young established populations are likely to have minimal effects on the invaded communities (West et al. Reference West, Matlaga, Muthukrishnan, Spyreas, Jordan, Forester and Davis2017). Therefore, targeting eradication efforts to newly invaded populations that have yet to negatively affect the native community is desirable whenever possible.
Finally, our estimates of Miscanthus eradication costs are likely conservative, because we only considered two habitats, of which only one was conducive to Miscanthus infestations. Many of the M. sinensis infestations reported on EDDMapS occur in open and disturbed habitats, including roadsides and forest and residential property edges (Bonin et al. Reference Bonin, Mutegi, Snow, Miriti, Chang and Heaton2017; Dougherty et al. Reference Dougherty, Quinn, Endres, Voigt and Barney2014; Hager et al. Reference Hager, Rupert, Quinn and Newman2015; Smith et al. Reference Smith, Tekiela and Barney2015). Therefore, it is hard to know how the eradication cost estimates within our selected habitats compared with alternative habitats that may or may not be more conducive to Miscanthus infestations.
Many factors influence the likelihood that an eradication program will or will not be successful, including traits of the invasive species, as well as environmental and socioeconomic factors (Dodd et al. Reference Dodd, Ainsworth, Burgman and McCarthy2015; Rejmánek and Pitcairn Reference Rejmánek and Pitcairn2002). Miscanthus species contain many traits that increase the feasibility of eradication. For example, they are easily detectable when at maturity and have a short-lived seedbank and somewhat limited distribution (mostly to open, disturbed habitats), and adequate methods of control have been established (Anderson et al. Reference Anderson, Voigt, Bollero, Hager, Bollero, Hager, Anderson and Voigt2010). Additionally, more than 86% of confirmed infestations on EDDMapS are less than 1 ha, an area in which eradication is generally successful (Rejmánek and Pitcairn Reference Rejmánek and Pitcairn2002), and all the infestations were less than 100 ha, an area in which previous work has deemed eradication success to be greater than 30%. Despite this, the probability of eradication within an area is unlikely if that species is currently under cultivation (Dodd et al. Reference Dodd, Ainsworth, Burgman and McCarthy2015). Because of the popularity of Miscanthus spp. as ornamental grasses within lawns and gardens, continued reintroduction within an area is likely.
Our research adds to the growing body of empirical studies monitoring eradication of an invasive plant (Rejmánek and Pitcairn Reference Rejmánek and Pitcairn2002; Scott et al. Reference Scott, Batchelor and Webber2019) with the aim of estimating the costs of eradication (Buddenhagen Reference Buddenhagen2006; Jardine and Sanchirico Reference Jardine and Sanchirico2018; Mangold et al. Reference Mangold, Fuller, Davis and Rinella2018; Tye Reference Tye2007) and can be used by economists to strengthen economic analyses on the true costs resulting from invasive species and their control efforts. Many studies use a post hoc approach to estimate eradication or control costs via periodic surveys to land managers to detail incurred expenses (Jardine and Sanchirico Reference Jardine and Sanchirico2018; Mangold et al. Reference Mangold, Fuller, Davis and Rinella2018). Within our study, we tracked Miscanthus survival while estimating the associated costs of management, which can enable more empirically informed decisions on the cost-benefit of management activities. For example, excavating the few remaining Miscanthus giganteus plants comprised 15% and 45% of the total eradication costs incurred at the TR and VRO sites, respectively, yet still did not result in full eradication. Was the extra effort to remove the few remaining Miscanthus plants worth the cost?
At what point is the economically optimal decision to switch from a goal of eradication to one of containment and maintenance management? Panetta (Reference Panetta2015) notes that controlling 90% of individuals within a population is generally cost-effective, but beyond that additional control is subject to the law of diminishing returns. Our data support that, as controlling the remaining 5% to 10% of Miscanthus individuals at the TR and VRO sites comprised between 30% and 60% of the total eradication costs incurred. While Miscanthus species can reduce species richness and diversity (Hager et al. Reference Hager, Rupert, Quinn and Newman2015), it is likely that their effects on native plant communities do not warrant the exorbitant time and resources required for eradication. Perhaps eradication of existing feral Miscanthus populations is not a reasonable or cost-effective goal, and continued management to contain feral populations is instead the economically optimal decision.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2022.20
Acknowledgments
We gratefully acknowledge the help of several research technicians and research assistants in implementing the eradication procedures at our study locations. The establishment of the original Miscanthus populations was funded by U.S. Department of Agriculture–National Institute of Food and Agriculture (USDA-NIFA) AFRI Project No. 2012-67013-19427. This work was supported by the USDA-NIFA and Hatch Appropriations under Project No. PEN04759 and Accession No. 1025327. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer. No conflicts of interest have been declared.






