Background
There is increasing recognition that developmental origins play an important role in epidemiology. Reference Barker1 According to the Developmental Origins of Health and Disease (DOHaD) hypothesis, exposures and events during preconception, prenatal, birth and early-life periods could affect an individual’s development and disease susceptibility. Reference Hanson and Gluckman2–Reference Warmink-Perdijk, Peters and Tigchelaar7 Beyond the DOHaD hypothesis, recent evidence suggests that prior exposures can be transferred across generations. Reference Arshad, Karmaus, Zhang and Holloway8,Reference Mørkve Knudsen, Rezwan, Jiang, Karmaus, Svanes and Holloway9 Children can inherit developmental programming across generations even when they have not been exposed to the environment that triggered the changes. Reference Sutton, Gilmore and Dunger10,Reference Padmanabhan, Cardoso and Puttabyatappa11 Thus, it is essential to consider the cross-generational factors when assessing the subject’s health risk. Therefore, understanding multigenerational relationships has profound implications for developing public health interventions to prevent diseases. Reference Hochberg, Feil and Constancia12
Unfortunately, conventional epidemiologic study designs cannot address intergenerational inheritance issues wel. Reference Duijts, Reiss, Brusselle and de Jongste13,Reference Harville, Breckner, Shu, Cooper and Bazzano14 Research estimating disease risk over an individual’s lifetime needs prospective observational data from multiple generations with long-term follow-ups. Reference Arshad, Karmaus, Zhang and Holloway8 Multigenerational cohorts are crucial to verify the above-mentioned effects among human subjects. Several multigenerational cohort studies are currently underway, such as the LifeLines Cohort Study, the Uppsala Birth Cohort, and the Framingham Heart Study. Several reviews of birth cohort studies have also been published. Reference Slade, Chapman, Swift, Keyes, Tonks and Teesson15–Reference Alduraywish, Lodge and Campbell20 However, birth cohort studies are structured to include a span of up to two generations, a factor that limits their ability to address the complexities of intergenerational inheritance. These studies have predominantly focused on pregnancies and fetuses, omitting male participants and lacking in the long-term follow-up necessary to include a wider range of age groups. This focus results in a narrower scope of research regarding exposures, outcomes, and research topics compared to multigenerational cohorts. In addition, reviews of birth cohort studies did not identify and present detailed and up-to-date information on multigenerational cohorts. They did not use a unified framework to categorize multigenerational cohort studies or summarize their characteristics.
Given this research gap, we carried out a scoping review on multigenerational cohort studies. We opted for a scoping review to systematically identify key concepts and describe major findings because a topic not extensively investigated is unsuitable for a systematic review. Reference Pham, Rajić, Greig, Sargeant, Papadopoulos and McEwen21,Reference Arksey and O’Malley22 This scoping review aims to map existing literature to summarize multigenerational cohort studies’ characteristics, issues, and implications and hence provide evidence to the DOHaD hypothesis and intergenerational inheritance. To identify multigenerational cohorts, we adopted a three-step search strategy: database searching with specific keywords, manual reference checks, and examination of cohort study databases to ensure comprehensive coverage. Conducting this scoping review is essential as it addresses the gap in understanding the long-term effects of early-life exposures on subsequent generations. This review is instrumental in clarifying the characteristics and implications of multigenerational cohorts, providing a crucial foundation for validating the DOHaD hypothesis, and informing future research and public health strategies aimed at disease prevention across generations.
Methods
This study followed Arksey and O’Malley’s five-stage scoping review framework. The five stages are identifying research questions, identifying relevant studies, selecting studies, charting data, and collating, summarizing, and reporting results. Reference Arksey and O’Malley22 The following summarizes our approach to each stage. This study followed Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) reporting guidelines. The supervisors scrutinized the study protocol before conducting the scoping review.
The research questions
We aimed to answer the following questions by conducting this scoping review:
-
How many multigenerational cohorts have been conducted in the world?
-
What are the characteristics of the multigenerational cohorts? For example, the basic information, categorization, exposures and outcomes, and data collected by the cohorts.
-
What are the differences between multigenerational cohorts and traditional cohorts? What are the advantages and disadvantages of multigenerational cohort studies? And what implications and insights have the multigenerational cohort studies brought?
Identifying relevant studies
We adopted a three-step search strategy to identify multigenerational cohorts comprehensively. Firstly, we searched PubMed, EMBASE, and Web of Science databases from the inception of each dataset to June 20th, 2022, to retrieve relevant articles. The search terms comprised five keywords: three-generation, multigenerational, intergenerational, transgenerational, and cohort. The complete search terms are shown in Appendix 1. Secondly, we manually searched the reference lists of included articles to identify further studies of interest. Finally, we explored cohort study databases (i.e., LMIC LPS Directory, JPND Global Cohort Portal, and Birthcohorts.net) to find related multigenerational cohorts that might have been overlooked in the previous two rounds.
Study selection
We are interested in multigenerational cohort studies rather than published articles. We first aimed to find corresponding articles, then identify multigenerational cohorts from included literature. Following are the inclusion and exclusion criteria for our scoping review:
Inclusion criteria:
-
Articles related to multigenerational cohorts.
-
Cohort profiles, primary research studies, reviews, meta-analyses, guidelines, and dissertations that included information of multigenerational cohorts.
-
Articles were not limited by study designs, population, interventions, outcomes, geographical locations, settings, and topics.
Exclusion criteria:
-
Multigenerational cohorts are generated through linked and registry data and without any own fieldwork.
-
Data on one or more generations were provided by family members instead of through direct enrollment of the participants themselves.
-
Publications of letters to editors, correspondence, points of view, ideas, opinions, magazine and newspaper articles, and case reports.
-
Articles were not published in English.
-
The full text was not accessible.
Records retrieved from databases were imported into the Covidence software for screening. Two reviewers (JT, ZFZ) independently screened titles and abstracts. After the reconciliation of any discrepancies, full texts of related articles were then retrieved and evaluated for eligibility. Then we identified the multigenerational cohort studies from included articles and their reference lists. Finally, we checked the online cohort study databases for missing cohorts. Any controversy was solved by consensus or consultation with a third reviewer (XLX).
Charting the data
We developed a data extraction form through the experienced reviewers’ consultation and pre-piloted the form to make sure all related data could be extracted. After identifying the multigenerational cohort studies, we searched these cohorts online and tried to synthesize the information we needed from accessible materials, including but not limited to the cohorts’ profiles, publications, and web pages. We retrieved data from the most recent and comprehensive publications if information differed between resources. We charted the following cohorts’ characteristics: the name of cohort, study design, country, sample size, time range, frequency of follow-up, participants, data collection strategies, exposures, and outcomes of included cohorts. Two reviewers (JT, ZFZ) independently extracted data. The consensus was reached through discussion.
Collating, summarizing, and reporting the results
-
We conducted a descriptive analysis mapping the characteristics of included multigenerational cohort studies, and the results were presented using tables and figures.
-
We also carried out a thematic summary describing the general properties of multigenerational cohorts, such as their advantages and disadvantages, differences between traditional cohorts, and prospects in the future.
-
Based on this scoping review and previous literature, Reference Kuriyama, Metoki and Kikuya23,Reference Felix, Joubert and Baccarelli24 we came up with a categorization scheme of existing multigenerational cohorts, and classified the included multigenerational cohort studies into four categories as population-based cohort extended three-generation cohort, birth cohort extended three-generation cohort, three-generation cohort, and integrated birth and three-generation cohort.
Results
The flowchart of this scoping review (Fig. 1) describes the results of screening and research selection processes. We found 2,752 records by database searching. After removing 1,399 duplicated records, 1,353 articles were eligible for the initial screening of titles and abstracts. Among these, 59 articles were determined to be qualified for full-text reviews. Ultimately, 11 multigenerational cohort studies were identified through electronic search. A further 17 eligible cohort studies were identified by a manual search of reference lists and cohort databases. Therefore, this scoping review identified 28 unique multigenerational cohort studies in total. The study characteristics of included multigenerational cohort studies are detailed in Appendix 2.

Figure 1. Flowchart of this scoping review.
Study design
Based on this scoping review and previous literature, Reference Kuriyama, Metoki and Kikuya23,Reference Felix, Joubert and Baccarelli24 we classified the included multigenerational cohort studies into four categories (Fig. 2). In general, a population-based cohort extended three-generation cohort was initiated as a population-based cohort with collected information from original participants (F0). As the cohort grew, the offspring of the original participants were recruited as F1 (F0’s children) and extended to F2 (F0’s grandchildren). Secondly, the birth cohort extended three-generation cohort was initiated as a birth cohort with collected information on pregnancies (F0) and their fetus (F1). As the cohort extended, F1’s children were recruited as F2. While the three-generation cohort was initiated with a three-generation study design at the very beginning stage, having a data collection strategy to collect information on F0 (grandparents), F1 (parents) and F2 (grandchildren) at the same stage. Finally, the integrated birth and three-generation cohort was initiated as a birth cohort with collected information on pregnancies (F1) and their fetus (F2) while integrating a three-generation study design with F0 (F1’s parents) information collected plan. As shown in Figure 3, among the included 28 multigenerational cohort studies, most cohorts (n = 15, 53%) were categorized as birth cohort extended three-generation cohort. Nine population-based cohorts extended three-generation cohorts (32%) and three three-generation cohorts (11%) in total. In comparison, only one study (4%) was identified as integrated birth and three-generation cohort.

Figure 2. Main exposures and outcomes of mutigenerational cohort studies, and the time course for different types of cohorts. F0: generation 1/grandparents; F1, generation 2/parents; F2, generation 3/children. Usually, population-based cohort extended three-generation cohort’s baseline started when F0 were adults, birth cohort extended three-generation cohort’s baseline started when F1 birthed, integrated birth and three-generation cohort’s baseline started when F2 birthed, and there generation cohort’s baseline started when F2 were juveniles.
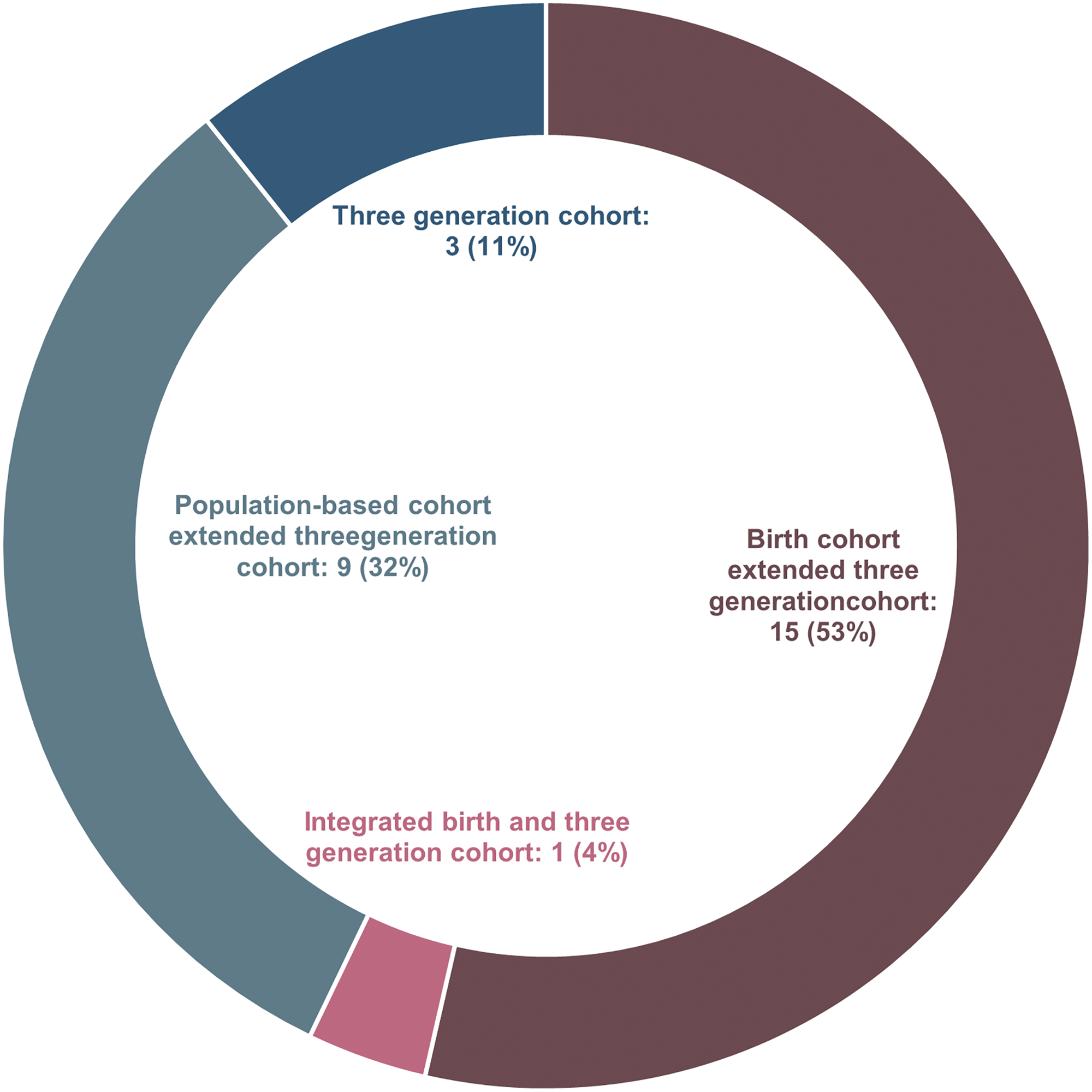
Figure 3. Category distribution of included multigenerational cohort studies.
Geography
The 28 multigenerational cohorts were conducted in 19 countries worldwide (Fig. 4). The majority of the cohorts (n = 6, 21%) were conducted in the United States, followed by the United Kingdom (n = 3, 11%), Australia (n = 3, 11%), Germany (n = 3, 11%), and the Netherlands (n = 2, 6%). The rest cohorts (n = 10, 37%) were from France, Japan, Sweden, Ireland, Brazil, Canada, Denmark, New Zealand, Filipino, and Israel, respectively. And one cohort (3%) conducted fieldwork in Northern Europe (Norway, Denmark, Sweden, Iceland, and Estonia), Spain, and Australia. We can see that most cohorts were conducted in Europe (n = 12, 43%) and North America (n = 7, 25%). There were fewer included cohorts from Oceania (n = 4, 15%) and Asia (n = 3, 12%). And only one study came from South America (4%). According to World Bank classification by income, most cohorts (n = 26, 93%) came from high-income countries. The remaining two cohorts were from middle-income countries (7%).
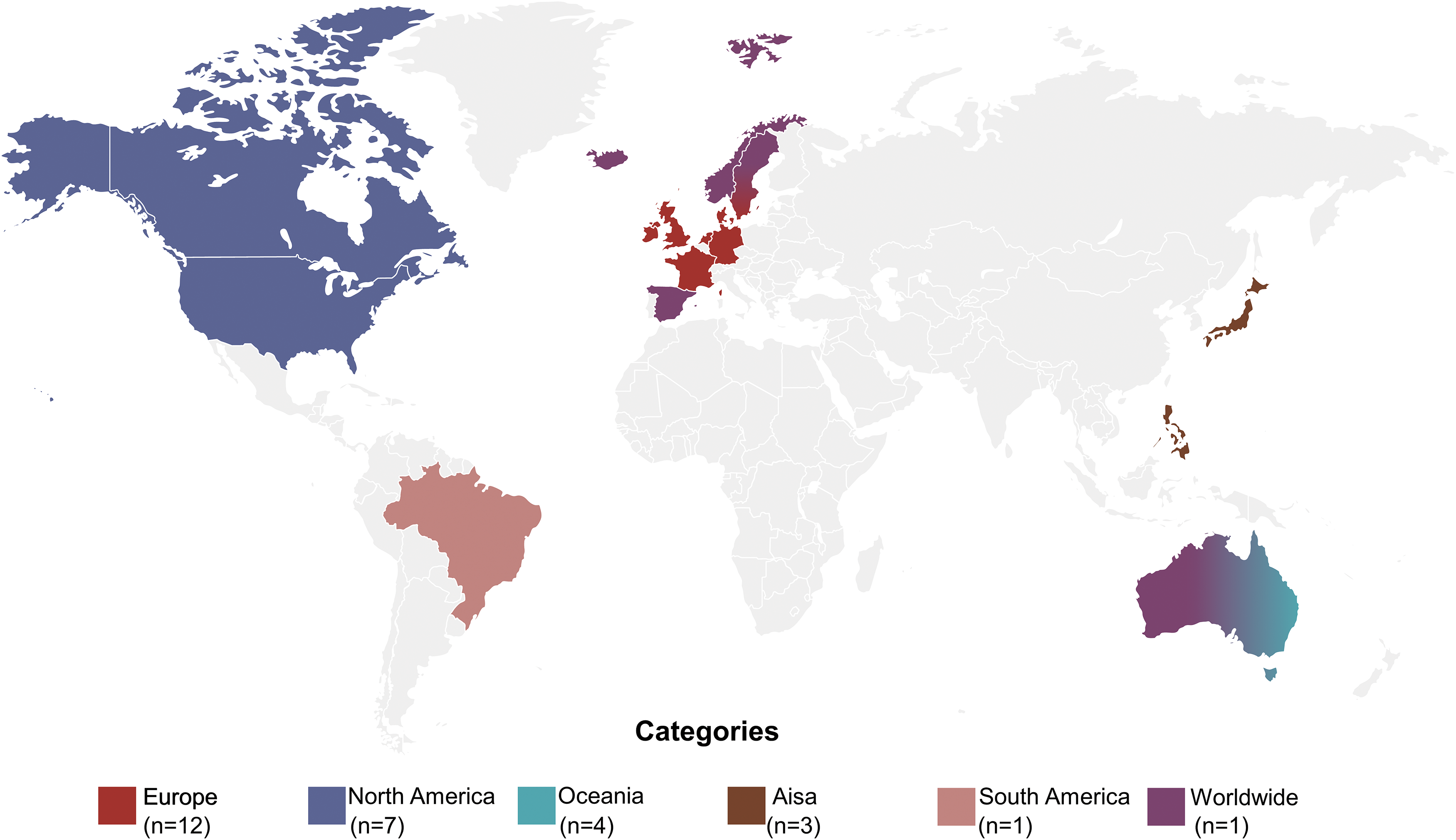
Figure 4. Geography distribution of included multigenerational cohort studies.
Time range and follow-up of F2
The study duration and follow-up of F2 differed by cohort. Each cohort adhered to its unique protocol, depending on its purposes, hypotheses, and available funding. Figure 5 demonstrates the included cohorts’ time range and cumulative years of follow-up of F2. The study duration of F2 ranged from two years (MUSP cohort) to 31 years (NCDS cohort). The earliest year of F2’s data collection was 1990 (PAS cohort); the most recent was 2016 (93Cohort-II and MUSP cohort). There were 11 cohorts (39%) that started the data collection of F2 between 2000 and 2010, and nine cohorts (32%) started after 2010. As for follow-up of F2, the shortest cumulative years of follow-up of F2 was six months from the MUSP cohort, while the longest follow-up of F2 was 20 years from the Nova Scotia 3G cohort. And the number of follow-up waves of F2 also varied across included cohorts. Most cohorts conducted less than five waves of data collection of F2 until now. While the Nova Scotia 3G cohort conducted over 20 waves of data collection of F2. Most of the cohorts still had ongoing data collection and follow-up.
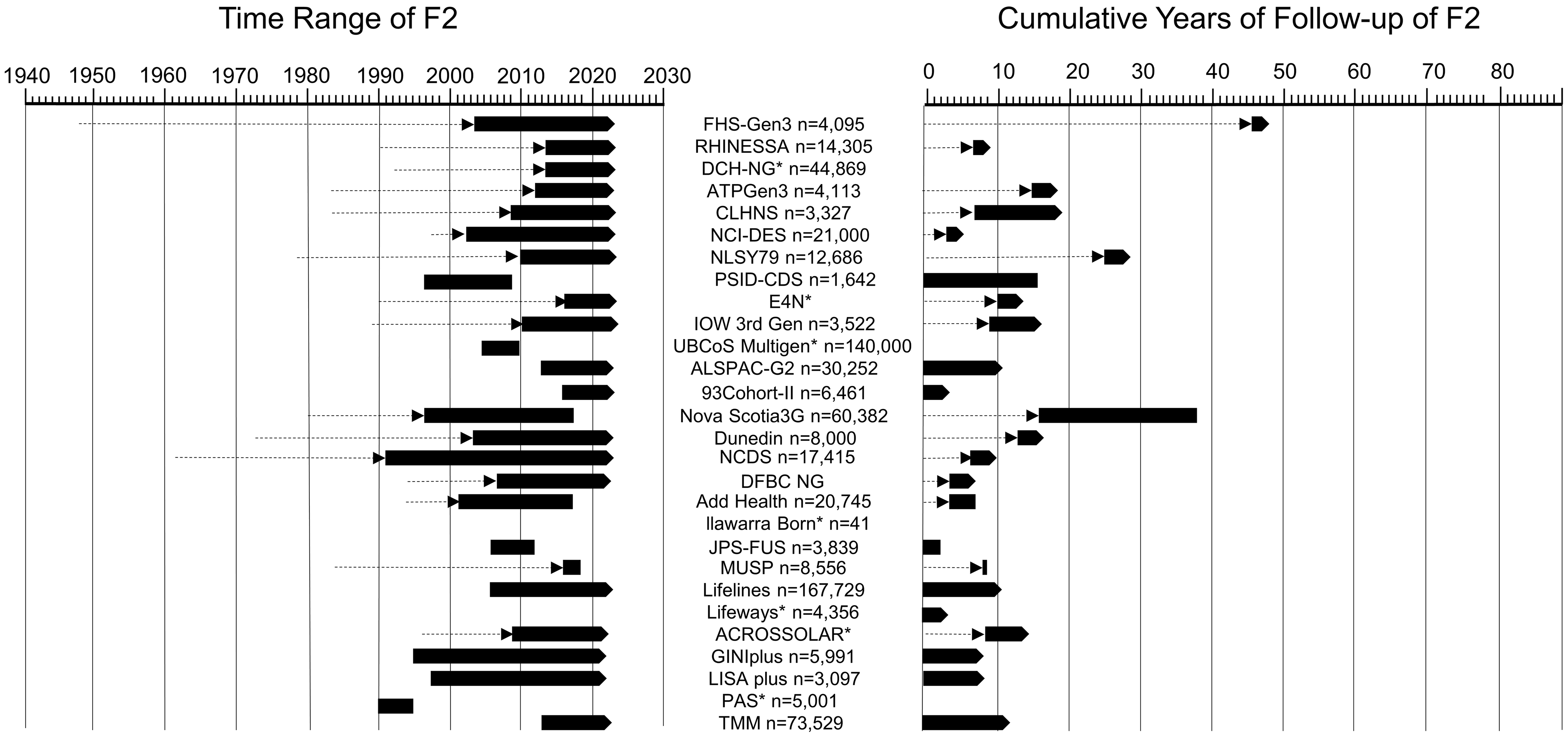
Figure 5. Time range and cumulative years of follow-up of F2. Dashed lines with arrow indicating F0 (generation 1/grandparents) and F1 (generation 2/parents), solid cubes indicating F2 (generation 3/children), solid cubes with arrow indicating the study is ongoing. * indicating the time range and/or cumulative years of follow-up of F2 were not given.
Sample size
The sample size of included cohorts varied largely from 41 to 167,729. Except for the Illawarra Born Cohort (4%) included only 41 participants, most of (n = 15, 53%) the included cohorts’ sample size was between 1,000 and 10,000, and the rest (n = 12, 43%) cohorts’ sample size was over 10,000. There are two cohorts’ sample sizes beyond 100,000: the Lifelines cohort (167,729) and the UBCoS Multigen cohort (140,000).
Participants
Although several cohorts were initiated very early and comprised up to five-generation participants, due to the loss of follow-up and a large variety of missing data from the previous generations, except for the Lifelines cohort included integrated four-generation participants’ information, the other 27 cohorts’ participants had three-generation information. Most cohorts’ participants were enrolled from one city of a country, such as Miyagi Prefecture in Japan, Uppsala in Sweden, and Framingham in the United States. However, the Lifelines cohort conducted their survey in the northern three provinces of the Netherlands, the NCDS cohort’s participants came from England, Scotland and Wales, and the RHINESSA cohort recruited participants from seven countries. Almost all studies sought to enroll individuals in the general population, excluded the NCI-DES cohort’s inclusion criteria were diethylstilbestrol exposed and unexposed mothers and their offspring, and the DFBC cohort recruited people who went through the Dutch famine and their offspring.
Data collection
Data collection of each cohort was summarized in Table 1. We classified the data into six categories following relevant references: Reference Felix, Joubert and Baccarelli24–Reference Townsend, Trabert and Fortner26 physical examination, general information, health status, lifestyle and environment, psychosocial parameters, and biomaterials and genomics. Usually, the data collected in F0 was consistently collected in both F1 and F2. Such as general information, health status, lifestyle, and environment have been collected continuously through three generations for all cohorts. But some cohorts modified their data collection strategy at F2 with either added or deleted aspects. For example, NLSY79 cohort and Illawarra Born cohort deleted psychosocial parameters; Add Health cohort deleted psychosocial parameters and biomaterials and genomics; PSID-CDS cohort added psychosocial parameters; IOW 3rd Gen cohort, Dunedin cohort, MUSP cohort, and 93Cohort-II cohort added biomaterials and genomics; JPS-FUS cohort added psychosocial parameters and biomaterials and genomics in F2 data collection.
Table 1. Summary of data collected by three generations of included multigenerational cohort studies a
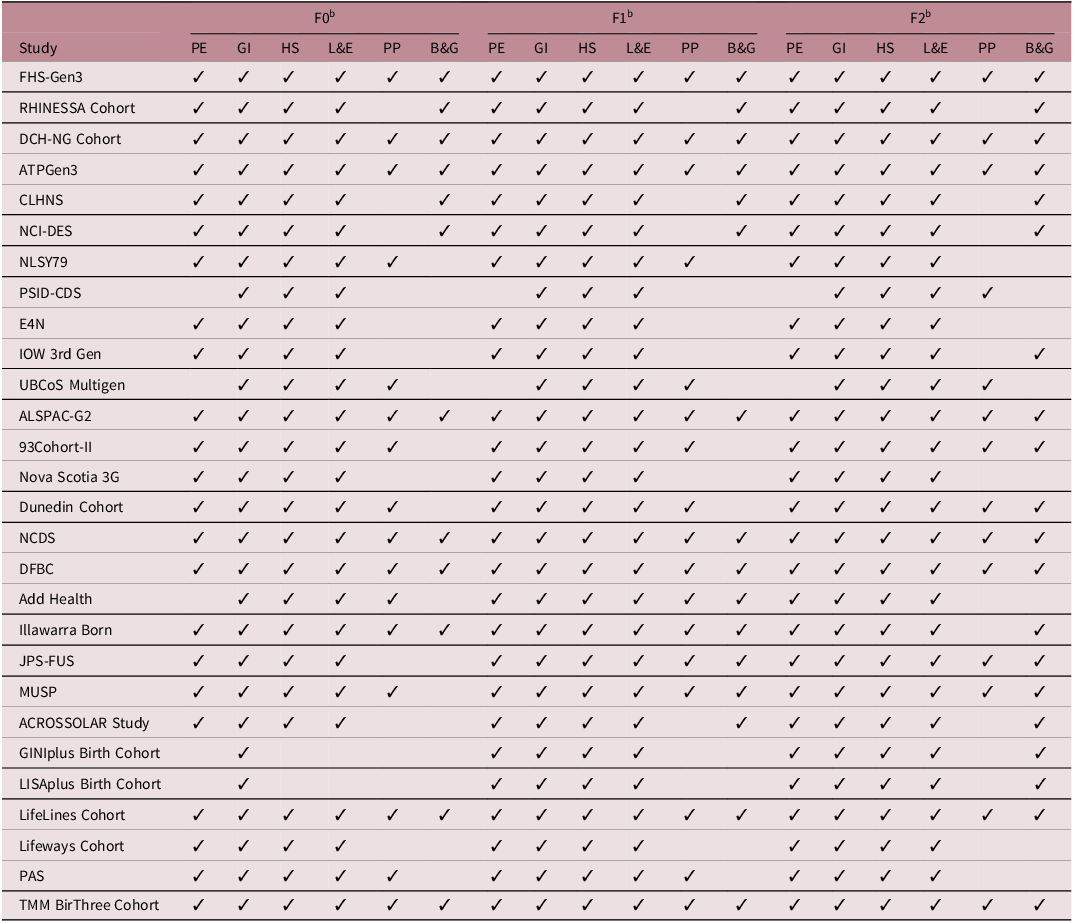
a Explanation of the collected data’s category
Physical Examination (PE): Anthropometry, blood pressure, pulmonary function, electrocardiogram, skin autofluorescence, neuropsychiatric health, cognition, etc.
General Information (GI): Demographics, socioeconomics, family composition, employment, education, income, etc.
Health Status (HS): Medical history, medication use, healthcare use, reproductive health, child birth and development, birth weight, etc.
Lifestyle and Environment (L&E): Physical activity, nutrition, diet, smoking, alcohol using, drug taking, sleep, physical environment, etc.
Psychosocial Parameters (PP): Depression, anxiety, quality of life, well-being, health perception, somatization, personality, stress, social support, independence, etc.
Biomaterials and Genomics (B&G): Blood sample, urine sample, DNA etc.
b F0: Generation 1/grandparents; F1: Generation 2/parents; F2: Generation 3/children.
Exposures
Across included multigenerational cohort studies, a large number of exposures and outcomes were adopted. Compared to traditional cohorts, the multigenerational cohort studies especially focus on F0 and/or F1 exposures and the corresponding F2 health outcomes. The main exposures of included multigenerational cohort studies are shown in Figure 6. The size of each rectangle in this tree map is proportional to the number of exposures from all included cohorts. Almost all cohorts had common exposures related to general information (demographics and socioeconomics such as age, education, employment, marital status, and income) and lifestyle and environment (cigarette and alcohol consumption, drug taking, physical activity, dietary and nutrition, physical environment). Also, many cohorts used collected health status information of F0 and/or F1 over the life course as exposures. Furthermore, psychosocial parameters (parental involvement, stressful life events, marital conflict, and periods of lone parenthood) and biomaterials and genomics (sex, race, and genetics) were frequently treated as exposures as well. Notably, among these collected data, reproductive factors (hormones, menopause, contraception, marital and fertility histories, mode of feeding) and childhood factors were often taken as exposures in some cohorts. In addition, a few cohorts investigated the natural events’ influence on three generations, such as the TMM BirThree cohort used earthquake and tsunami disasters, the ALSPAC-G2 cohort used major changes that have occurred over the last 20–25 years, and the Dutch Famine Birth Cohort Study used famine as exposures.
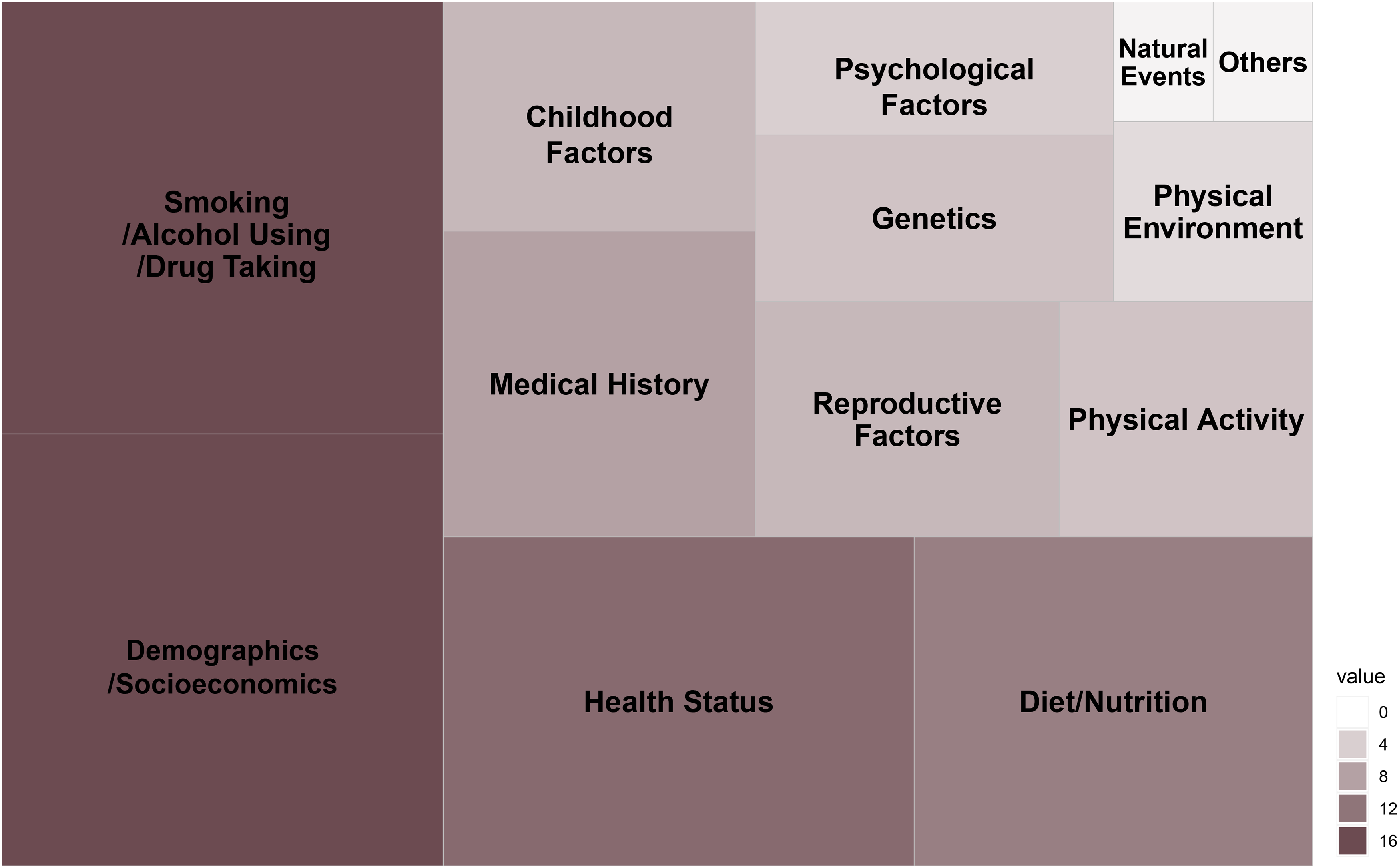
Figure 6. Summary of main exposures of included multigenerational cohorts. Size of rectangle is proportional to the number of exposures from included cohorts.
Outcomes
Figure 7 displays the main outcomes of included multigenerational cohort studies. The size of each rectangle in this tree map is proportional to the number of outcomes from all included cohorts. The multigenerational cohort studies usually took intergenerational inheritance of diseases as the outcome. The most frequently investigated diseases were obesity, cardiovascular diseases (stroke, heart failure, angina pectoris, myocardial infarction, coronary heart disease, and atrial fibrillation), and child health (low birth weight of infancies, child physical and/or mental development). Followed by mental health (depression, anxiety, autism, post-traumatic stress disorder, suicide), respiratory health (asthma, chronic obstructive pulmonary disease), diabetes mellitus, and hypertension. Besides, cancers (breast cancer, ovarian cancer, prostate cancer, endometrial cancer), cognition function (dementia), reproductive health (pre-eclampsia, gestational hypertension, endometriosis), allergic disease (atopic dermatitis, eczema, rhinitis, food allergy), and social inequality were also taken as outcomes by some cohorts. Few studies also investigated more specific diseases, such as headaches and oral health.

Figure 7. Summary of main outcomes of included multigenerational cohorts. Size of rectangle is proportional to the number of outcomes from included cohorts.
Topics
Overall, most of the included multigenerational cohort studies are population-based and have collected vast amounts of data on many domains which generally covered the exposures and outcomes of those cohorts. They explored the environmental, socioeconomic, lifestyle, physiological, metabolic, genomic, and/or epigenomic contributions to health across the life course and generations and boosted verification of the DOHaD hypothesis. Still, some studies have a particular focus, such as cardiovascular diseases (FHS-Gen3 cohort), lung health (RHINESSA cohort), diethylstilbestrol (NCI-DES cohort), famine (DFBC cohort), and cardiometabolic risk (JPS-FUS cohort). Specially, the UBCoS Multigen cohort, 93Cohort-II cohort and MUSP cohort took health inequalities as one of their topics. Despite their various topics and focus, most studies aim to investigate the disentanglement of genetic, lifestyle, and environmental influences on disease development and to study the between-generation similarities.
Discussion
Principal findings
In this scoping review, we identified 28 unique multigenerational cohort studies and categorized them into four types: population-based cohort extended three-generation cohort, birth cohort extended three-generation cohort, three-generation cohort, and integrated birth and three-generation cohort. The included 28 multigenerational cohorts were conducted in 19 countries around the world. Most studies were conducted in the United States (n = 6, 21%). The sample size of included cohorts varied largely from 41 to 167,729. The study duration ranged from two to 31 years, and the follow-up of cohorts also differed. A majority of cohorts have comprehensive data collection schemes. Almost all cohorts had common exposures to socioeconomic factors, lifestyle, and F0 or F1’s health and risk behaviors over the life course. These cohorts usually took intergenerational inheritance of diseases as the outcomes, and the most frequently investigated outcomes were obesity, child health, and cardiovascular diseases. Despite their various topics and focus, most studies aim to investigate the disentanglement of genetic, lifestyle, and environmental influences on disease development and to study the between-generation similarities.
SWOT analysis of multigenerational cohort studies
Strength
The main strength of multigenerational cohorts is the long-term follow-up of a cohort with a repeated collection of various data from multidisciplinary topics, making it possible to understand how different risk factors affect one’s disease susceptibility not just in one period of life but also cumulative over time even across generations. Also, the multigenerational cohort study design has statistical strengths regarding its accuracy, various levels of data, separating genetic and environmental factors, and direct haplotype assessment. Reference Stolk, Rosmalen and Postma25 Then, this type of study design provides extraordinary opportunities to study social characteristics such as socioeconomic mobility, partner preferences, and generation similarities, and also offers practical benefits in efficiency and a relatively high response rate. Furthermore, the broad age range of participants included in the multigenerational cohorts allows for early detection of events before it’s too late, hence broadening insights into time-dependent effects, and can examine how various exposures affect disease development at different ages.
Weakness
A major problem of multigenerational cohort studies is the loss of follow-up of the cohort over time which is nearly inevitable for studies that are designed to consecutively recruit more than two generations. Reference Arshad, Karmaus, Zhang and Holloway8 Due to the long-term follow-up and attrition of the cohort, multigenerational cohort studies are likely to have incomplete measurements across generations and missing informative data. Also, the poor quality of some cohorts was mostly caused by the practical difficulties when collecting data across multiple generations. Another significant disadvantage of this study design is the generalizability of research findings. The participants of these cohorts are usually recruited from one location with certain ethnic people, and this cohort’s socioeconomic status and common exposures may differ greatly from other cohorts.
Opportunity
Multigenerational cohort studies are important for understanding the DOHaD. Only a small part of the familial clustering of phenotypes can be explained by traditional genetics, which implies the necessity to investigate additional underlying causes and mechanisms. Reference Manolio, Collins and Cox27 Although environmental and behavioral factors are also highly related to families, Reference Taouk and Schulkin28–Reference Karatsoreos, Thaler, Borgland, Champagne, Hurd and Hill30 recent studies in epigenetics indicate potential routes of multigenerational effects may be plausible. Reference Youngson and Whitelaw31,Reference Heindel, McAllister, Worth and Tyson32 Multigenerational cohort studies also provide unique possibilities for researchers to identify pre-conceptional influences on the next generation and the interaction between genetic and environmental factors. Reference Taouk and Schulkin28 Additionally, various topics can be involved and many scientific questions can be addressed in one multigenerational cohort with comprehensive data collected in this cohort.
Threat
Conducting multigenerational human cohort studies is difficult. Retrospective cohort studies are vulnerable to recall bias, Reference McGee, Weisskopf, Kioumourtzoglou, Coull and Haneuse33 and prospective cohort studies are hard to conduct as well since they require long-term follow-up and a large financial investment. And both these two kinds of cohorts are prone to miss data which is the common disadvantage of long-term cohorts. Reference Harville, Kruse and Zhao34 Even if the data is collected regularly, the critical periods of events or disease development usually can’t be identified under the analysis of general statistical methods. The independent effects are also difficult to determine due to inevitable measurement errors. Reference Hallqvist, Lynch, Bartley, Lang and Blane35 Furthermore, the explanation of research findings of multigenerational human cohort studies is complicated. Although it is easy and common to attribute to maternal inheritance, various epigenetic and transgenerational effects have been demonstrated to be paternal inheritance. Reference Zambrano, Martínez-Samayoa and Bautista36
Interpretation
For decades, evidence demonstrating that inherent properties can be transmitted across more than two generations has changed our knowledge of genetics and disease susceptibility theories. Reference Fitz-James and Cavalli37,Reference Bošković and Rando38 Pioneering research on humans revealed that exposure to smoking, famine, endocrine disruptors, or trauma could influence two to three generations’ offspring, which prompted a significant change in people’s view of heredity. Reference Accordini, Calciano and Johannessen39–Reference Greenblatt-Kimron, Shrira, Rubinstein and Palgi44 However, multigenerational cohort studies conducted on humans were limited, especially for transgenerational inheritance studies. Reference Arshad, Karmaus, Zhang and Holloway8,Reference Stegemann and Buchner45 Particularly, only a few studies begin at an early-life stage and sustain long term. Although child–mother pairs are usually recruited for birth cohort studies, Reference Géa-Horta, Silva Rde, Fiaccone, Barreto and Velásquez-Meléndez46–Reference Dearth-Wesley, Gordon-Larsen, Adair, Zhang and Popkin48 and grown-up children from existing cohorts may be recruited in other new cohorts, Reference Kannel, Feinleib, McNamara, Garrison and Castelli49–Reference Dougan, Willett and Michels51 rarely are cohorts that include integrated and completed three-generation data. Reference Harville, Breckner, Shu, Cooper and Bazzano14
Although there’s limited evidence of intergenerational and transgenerational inheritance for humans at present, some outstanding findings need to be noticed. For instance, a Swedish study found that providing proper nutrition to paternal grandparents when they were ten years old could decrease cardiovascular diseases and diabetes mellitus risk Reference Kaati, Bygren and Edvinsson52 and extend lifespan Reference Bygren, Kaati and Edvinsson53 of their grandchildren. There were also studies claimed that grandparents’ obesity condition might have an impact on grandchildren’s obesity either directly by grandparents to shape grandchildren’s behavioral decisions or indirectly by their parents. Reference Li, Adab and Cheng54,Reference Kanmiki, Fatima and Mamun55 Golding et al. demonstrated that when both grandmother and mother had smoked, compared to mothers who had not smoked, the smoking ones’ female descendant had declined in height, weight, and fat/lean/bone mass. Reference Golding, Northstone, Gregory, Miller and Pembrey56 And the Framingham Heart Study demonstrated that grandparents who had hypertension in early life could increase the hypertension risk among grandchildren after adjusting for parental confounding factors. Reference Niiranen, McCabe and Larson57 In addition, studies revealed that coronary heart disease, birth weight, body mass index, and major depressive disorder have intergenerational inheritance and can transmit across three generations. Reference Emanuel, Filakti, Alberman and Evans58–Reference Weissman, Berry and Warner62
Implications for policy and future research
The underlying practical benefits of establishing and verifying intergenerational and transgenerational inheritance are significant as we can better understand the determinants of major public health problems and hence formulate feasible and efficient screening and prevention strategies to reduce the disease burden. To deeply explore intergenerational and transgenerational inheritance, more animal experiments and human multigenerational cohort studies are required. Reference Mørkve Knudsen, Rezwan, Jiang, Karmaus, Svanes and Holloway9 Specifically, well-designed prospective multigenerational cohorts with large sample sizes can avoid many confounding factors and get high-quality results. Also, the collaboration between cohorts or meta-analysis of existing cohorts can synthesize current findings and provide potential new insight into DOHaD. And to determine the mechanisms of intergenerational and transgenerational inheritance, animal models, and human cohorts with more than three generations and up to F3 are needed. Reference van Steenwyk, Roszkowski, Manuella, Franklin, Mansuy and Skinner63
Strengths and limitations
Scoping reviews are comprehensive but not exhaustive enough when identifying and synthesizing the literature, Reference Levac, Colquhoun and O’Brien64 keeping a balance between the breadth and depth of study analysis. Reference Pham, Rajić, Greig, Sargeant, Papadopoulos and McEwen21 They offer an overview of existing literature irrespective of its quality, which is broader and more contextual than systematic reviews. Reference Pham, Rajić, Greig, Sargeant, Papadopoulos and McEwen21,Reference Arksey and O’Malley22,Reference Peters, Godfrey, Khalil, McInerney, Parker and Soares65
There are also some other limitations to our scoping review. First, we might not have captured all relevant multigenerational cohort studies. Nevertheless, our search strategy and the inclusion and exclusion criteria are systematic and thorough. Second, we did not formally assess the quality of the included studies, and quantitative data synthesis was not feasible either. Third, our review included only studies published in English. Studies published in other languages are worth reviewing in future research.
Conclusion
We identified 28 unique multigenerational cohort studies and proposed a four-type categorization scheme. The sample size, study duration, and follow-up of cohorts differed. Most cohorts have comprehensive data collection schemes, and a large number of exposures and outcomes were investigated. Most studies aim to disentangle genetic, lifestyle and environmental contributions to the development of diseases across generations.
This scoping review provides evidence for the potential implications of multigenerational cohort studies on the developmental origins of health and disease and intergenerational inheritance. We call for more research on large multigenerational well-characterized cohorts, up to four or even more generations, and more studies from low- and middle-income countries.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2040174424000035.
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary files.
Acknowledgments
None.
Author contribution
Jie Tan and Zifang Zhang are contributed equally.
Jie Tan: Investigation, Methodology, Writing the original draft. Zifang Zhang: Investigation, Software, Visualization. Xiaolin Xu: Conceptualization, Supervision. Lijing L. Yan: Writing – review and editing.
Financial support
This study was funded by grants to XX from China Medical Board Open Competition Program (21-416).
Competing interests
None.










