Introduction
Approximately 5%–8% of pregnancies worldwide are complicated by hypertensive disorders of pregnancy (HDP) including gestational hypertension (GH), characterised by new onset hypertension at or after 20 weeks’ gestation, and preeclampsia (PE), characterised by hypertension with associated maternal organ dysfunction or fetal compromise. Reference Umesawa and Kobashi1,Reference Brown, Magee and Kenny2 Intrauterine exposure to HDP has been associated with long-term health implications for children including increased risks of mild cognitive impairment, Reference Tuovinen, Eriksson, Kajantie and Räikkönen3 autism spectrum disorder and other neurodevelopmental disorders. Reference Maher, O’Keeffe and Kearney4 However, there is uncertainty regarding the impact of HDP on psychomotor development in infancy (birth to 2 years), a critical period of rapid development.
Studies have demonstrated increased risks, no difference, or even decreased risks of impaired motor or cognitive development after HDP exposure compared to infants of normotensive pregnancies (NTP). Reference Vakil, Henry, Craig and Gow5 These discrepant findings may be attributed to the non-standard adjustment of perinatal confounders, the differing use of developmental screening and assessment tools, and differing study cohorts, with some cohorts only including infants born small for gestational age (SGA) or preterm. SGA and preterm birth are independent risk factors for poor infant neurodevelopment that may confound the impacts of HDP exposure. Reference Thomaidis, Zantopoulos and Fouzas6
Thus, this cohort study aimed to determine the impact of HDP exposure on infant neurodevelopment by comparing neurodevelopmental screening and assessment outcomes at 6 months and 2 years between infants exposed to PE or GH versus NTP. Furthermore, we aimed to assess whether any impacts of HDP exposure were independent of SGA or prematurity status, and sociodemographic characteristics including parental education levels. Understanding the early impacts of HDP exposure may highlight opportunities for early screening, intervention and optimisation of child neurodevelopment in these potentially at-risk populations.
Method
Study design and setting
This is a sub-study of the prospective, single-centre cohort study, the P4 (Postpartum Physiology, Psychology and Paediatric) follow-up Study at a metropolitan tertiary care hospital in Sydney, Australia. The study was approved by the South-Eastern Sydney Local Health District Human Research Ethics Committee (HREC/12/POWH395). A detailed study protocol has been published, Reference Davis, Roberts and Mangos7 in addition to maternal physiological, Reference Brown, Roberts and Hoffman8,Reference Henry, Mangos and Roberts9 metabolic, Reference McLennan, Henry and Roberts10 mental health Reference Roberts, Henry, Harvey, Homer and Davis11 and infant growth Reference Gow, Roberts and Henry12,Reference Gow, Vakil and Roberts13 outcomes. This study was written with the STROBE reporting guidelines.
Participants
Study participants were infants of mothers in the P4 study. Mothers were eligible if they gave birth to a live singleton without major congenital abnormalities between January 2013 and December 2018, and had a good understanding of written and spoken English. Women were excluded if, prior to pregnancy, they had diabetes, hypertension, renal or other serious disease. Written informed consent for mother and baby was obtained at study enrolment by 6 months postpartum. The P4 study recruited 415 women who had either NTP (n = 302), PE (n = 90) or GH (n = 23), according to the International Society for the Study of Hypertension in Pregnancy Guidelines. Reference Brown, Magee and Kenny2 GH was defined as persistent, new onset hypertension (blood pressure ³140 mmHg systolic or ³90 mmHg diastolic) at or after 20 weeks’ gestation without features of PE, while PE was GH accompanied by proteinuria or other maternal organ dysfunction including acute kidney injury, liver, neurological or haematological complications, or uteroplacental dysfunction. Reference Brown, Magee and Kenny2 This study includes infants exposed to NTP, GH or PE who had completed the 2-year Ages and Stages Questionnaires, Third Edition (ASQ-3) Reference Squires, Twombly, Bricker and Potter14 or the 2-year Bayley Scales of Infant and Toddler Development, 3rd Edition (BSID-III) Reference Bayley15 assessment (Fig. 1). Preterm birth was defined as < 37 weeks’ gestation, Reference Brown, Magee and Kenny2 and SGA status as birthweight z-score corrected for sex and gestational age < -1.28, calculated for term infants using the World Health Organisation Child Growth Standards, Reference de Onis16 and for preterm infants using the INTERGROWTH-21st Preterm Postnatal Growth Standards. Reference Villar, Giuliani and Bhutta17
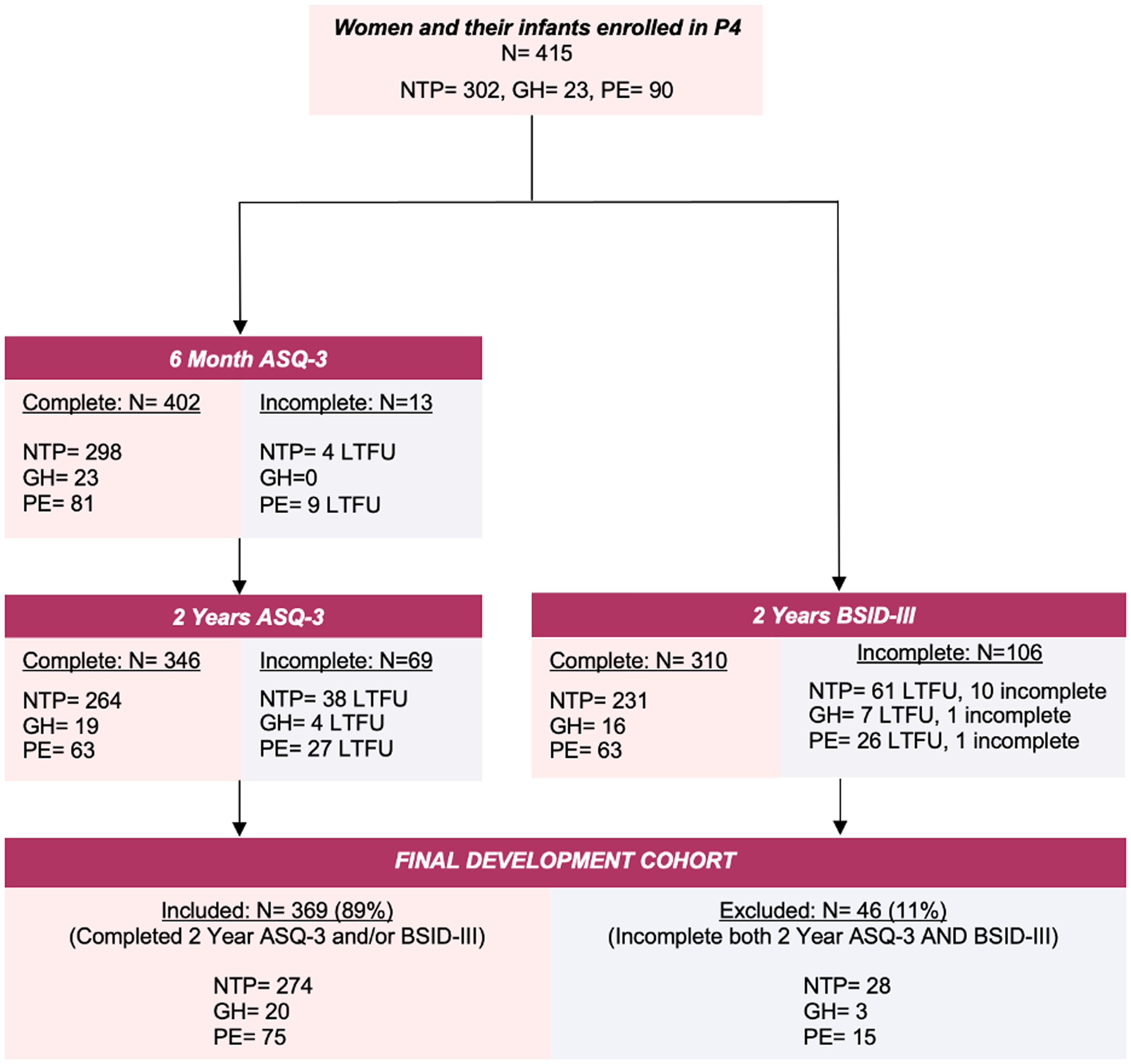
Figure 1. Flow diagram of study cohort. Abbreviations: ASQ-3, Ages and Stages Questionnaires, Third Edition Reference Squires, Twombly, Bricker and Potter14 ; BSID-III, Bayley Scales of Infant and Toddler Development, 3rd Edition Reference Bayley15 ; GH, gestational hypertension; LTFU, loss to follow up; N, number; NTP, normotensive pregnancy; PE, preeclampsia.
Data collection
Maternal sociodemographic information, medical history and birth details were collected retrospectively from the mother’s electronic medical record, and prospectively from P4 surveys (Supplementary Material S1) and study visits at 6 months and 2 years postpartum. Maternal blood pressure, body composition, and metabolic markers were also collected using standard methods at these study visits. Infant health details were collected by the paediatrician and parent surveys. Infant development was screened using the parent-completed ASQ-3 questionnaire at 6 months and 2 years (corrected for gestational age). Infant development was assessed by a child psychologist using the month-appropriate BSID-III assessment at approximately 2 years (corrected for gestational age). Testers were not blind to group status.
Outcomes
ASQ-3 scores at 6 months and 2 years were categorised as passes (development on schedule or close to cut-off requiring monitoring) or fails (indicating developmental risk and requiring formal developmental assessment) according to domain-specific cut-off values. Reference Squires, Twombly, Bricker and Potter14 Primary ASQ-3 outcomes included the proportion of infants who failed each ASQ-3 domain (communication, gross motor, fine motor, problem solving, personal-social) and proportion of infants who failed any ASQ-3 domain overall. The presence of developmental delay in a BSID-III domain was defined as performance > 2 standard deviations (SD) below the mean, Reference Bellman, Byrne and Sege18 corresponding to BSID-III scaled scores of ≤ 4, or performance ≤ 2nd percentile. Reference Bayley15 Primary BSID-III outcomes compared between exposure groups included BSID-III scaled scores in each domain, proportion of infants with developmental delay in each domain, and proportion of infants with developmental delay in any BSID-III domain overall.
Covariates
Maternal covariates considered in relation to HDP exposure and infant development included: age, parity, lifetime smoking history, maternal and paternal ethnicity (self-reported), tertiary education completion, first trimester weight and BMI, other pregnancy complications including medical, such as gestational diabetes mellitus, obstetric, such as antepartum haemorrhage, and fetal, such as reduced fetal movements, and at 6 months and 2 years; maternal blood pressure, alcohol consumption and Edinburgh Postnatal Depression Scale (EPDS) scores. Birth covariates included labour onset and mode of birth. Infant covariates included sex, birth gestation, prematurity status, SGA status, length of neonatal intensive care unit or special care nursery (NICU or SCN) stay, and total months breastfed in the first 2 years.
Statistical analysis
Descriptive statistics were used to summarise maternal, infant and birth covariates in each exposure group (NTP, GH or PE), as well as the excluded cohort. Developmental outcomes including ASQ-3 domains failed, BSID-III scaled scores and developmental delay in each BSID-III domain were compared between groups using independent samples T-tests and one-way ANOVA tests (parametric continuous data), Kruskal-Wallis tests (non-parametric continuous data), with Tukey post-hoc analysis to account for multiple comparisons, and Chi-Square or Fisher’s exact tests (categorical data). Associations between infant psychomotor development and covariates were explored using multivariable linear regression and binary logistic regression, including adjustment for known determinants of infant neurodevelopment such as SGA status, prematurity status, maternal education, maternal employment, maternal EPDS scores and other pregnancy complications. Statistical analysis was performed using IBM SPSS Statistics, version 26.0 (Chicago, IL). A two-tailed p-value of 0.05 was considered statistically significant in univariate analyses. In logistic regression models, Bonferroni corrections were applied to correct for multiple comparisons.
Results
Of the 415 infants initially enrolled in the P4 study, 369 (NTP = 274, 91%; GH = 20, 87%; PE = 75, 83%) had completed at least one or both the 2-year ASQ-3 surveys (n = 346), or 2-year BSID-III assessments (n = 310) (Fig. 1).
Table 1 details the parental demographic, maternal health, birth and infant health information of included participants. There were no statistically significant differences in parental demographic, maternal health, birth and infant health outcomes between retained participants and those lost to follow up. Compared to the NTP group, GH and PE mothers had poorer measures of postpartum physical health including higher blood pressure and insulin resistance. Compared to the NTP group, GH and PE mothers were more likely to give birth via elective or emergency caesarean section and have been nulliparous in the index pregnancy. Compared to other groups, PE-exposed infants were more likely to be born preterm, SGA, be admitted to a NICU or SCN, and were breastfed for a shorter duration.
Table 1. Parental demographic, maternal health, birth and infant health details of infants with intrauterine exposure to a normotensive pregnancy, gestational hypertension or preeclampsia
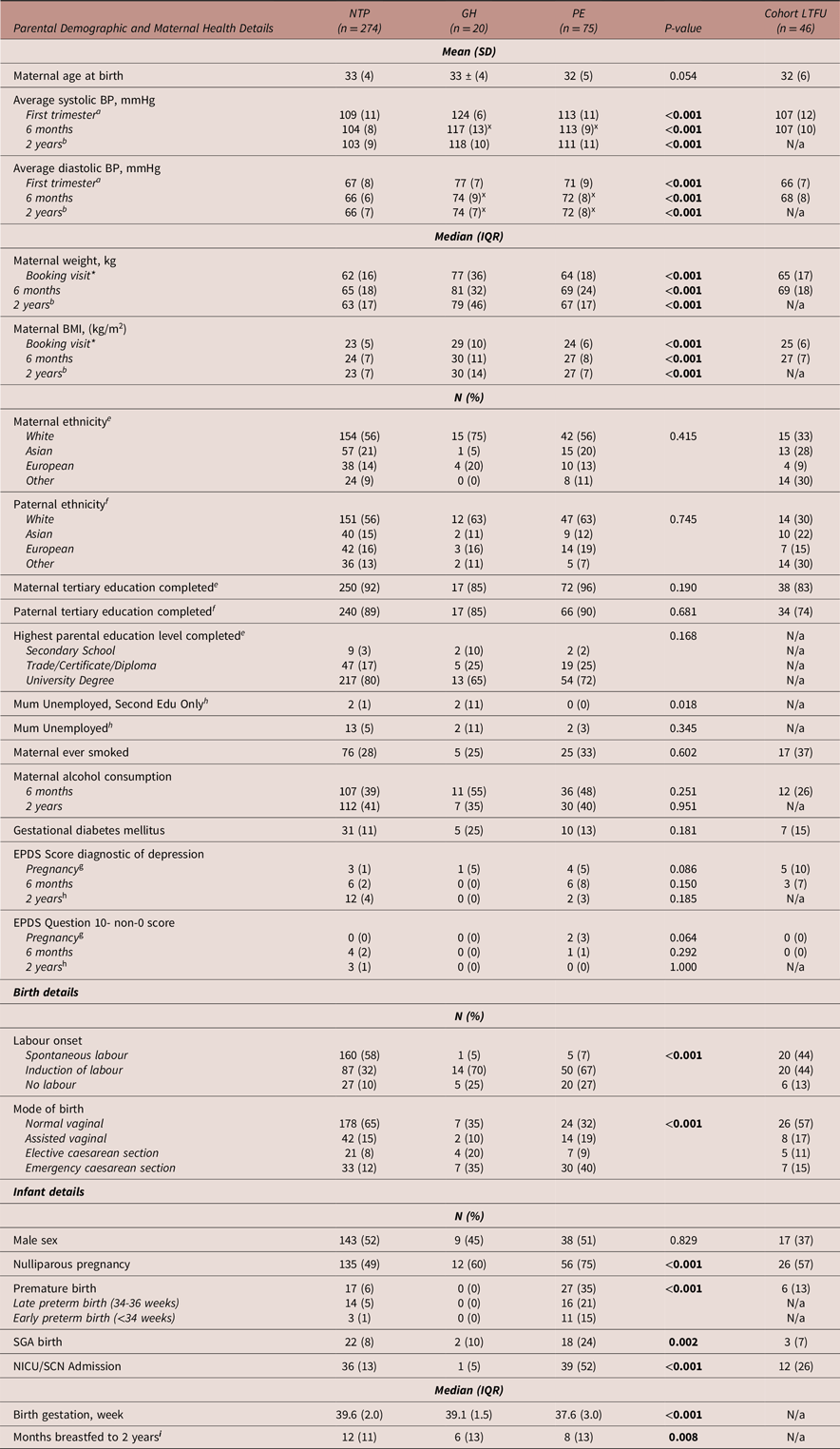
Abbreviations: BMI, body mass index; BP, blood pressure; EPDS, Edinburgh Postnatal Depression Score; GH, gestational hypertension; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; IQR, interquartile range; kg, kilograms; LTFU, loss to follow up (therefore excluded from further analyses); m, metres; mmHg, millimetres of mercury; N, number; N/a, not assessed due to lack of data/LTFU; NICU/SCN, neonatal intensive care unit/special care nursery; NTP, normotensive pregnancy; PE, preeclampsia; SD, standard deviation; SGA, small for gestational age. *Median (IQR) gestation of booking visit = 13 (3) weeks. xGH and PE groups did not significantly vary but were significantly greater than the NTP group. Missing data: a n = 60, b n = 176, c n = 4, d n = 181, e n = 1, f n = 6, g n = 27, h n = 8, i n = 14.
Univariate analyses
Table 2 details 6-month and 2-year ASQ-3 developmental outcomes. Compared to NTP or GH-exposed infants, a higher proportion of PE-exposed infants failed 4 of 5 domains, and at least 1 ASQ-3 domain at 6 months (NTP = 18%, GH = 5%, PE = 28%, p = 0.07), however this difference was not statistically significant.
Table 2. Infant ASQ-3 outcomes after intrauterine exposure to a normotensive pregnancy, gestational hypertension or preeclampsia
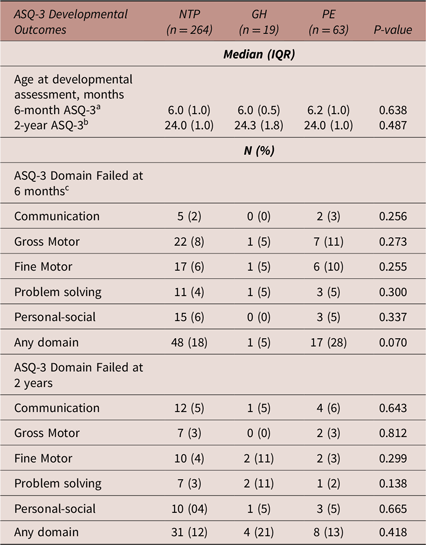
Abbreviations: ASQ-3, Ages and Stages Questionnaires, Third Edition; GH, gestational hypertension; IQR, interquartile range; N, number; NTP, normotensive pregnancy; PE, preeclampsia. Missing data: a n = 26, b n = 43, c n = 6.
Table 3 details 2-year BSID-III developmental outcomes. GH-exposed infants had on average lower BSID-III scaled scores in all domains, and although approaching significance in the expressive communication domain (p = 0.053), these differences were not statistically significant, and their scores were not indicative of developmental delay in any domain. There were no significant differences noted in the PE comparisons.
Table 3. Infant BSID-III outcomes after intrauterine exposure to a normotensive pregnancy, gestational hypertension or preeclampsia
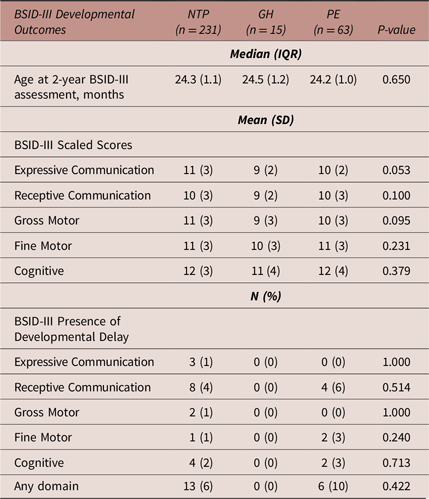
Abbreviations: BSID-III, Bayley Scales of Infant and Toddler Development, 3rd Edition; GH, gestational hypertension; IQR, interquartile range; N, number; NTP, normotensive pregnancy; PE, preeclampsia.
Multivariable analyses
Table 4 details binary logistic regression models exploring the association between PE and GH exposure compared to NTP exposure with 2-year ASQ-3 domain failure. When adjusting for prematurity status, SGA status, maternal tertiary education completion rates, maternal employment status, maternal EDPS scores at 2 years and other maternal pregnancy complications, GH was significantly associated with an increased risk of failing the problem-solving domain (p = 0.048). However, when corrected for multiple comparisons, this finding was no longer significant. Prematurity status was significantly associated with failing any domain (Exp(B) [95%CI]: 3.35 [1.27–8.88], p = 0.015).
Table 4. Binary logistic regression: adjusted model of the associations between preeclampsia and gestational hypertension compared to normotensive pregnancy exposure versus 2-year ASQ-3 domain failure
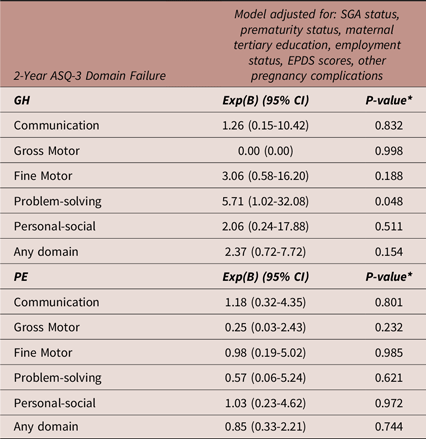
Abbreviations: ASQ-3, Ages and Stages Questionnaires, Third Edition; CI, confidence interval; Exp(B), exponent beta; GH, gestational hypertension; N, number; PE, preeclampsia, SGA, small for gestational age. Other pregnancy complications included gestational diabetes mellitus, preterm premature rupture of the membranes, antepartum haemorrhage, maternal infection, reduced fetal movements, polyhydramnios, oligohydramnios, exacerbation of Chron’s disease, cholestasis, maternal surgery during pregnancy. N = 346 for all models. *A p-value of 0.016 was considered statistically significant (Bonferroni correction).
Table 5 details multivariable linear regression models (same factors adjusted as for the 2-year ASQ-3) exploring the association between PE and GH exposure compared to NTP exposure with 2-year BSID-III scaled scores. Neither GH nor PE were significantly associated with lower BSID-III scaled scores at 2 years. Prematurity status was significantly associated with decreased scores in receptive communication (Exp(B) [95%CI]: −1.34[−2.63 - −0.04], p = 0.043) and cognitive domains (Exp(B) [95%CI]: −1.54[−2.90 - −0.19], p = 0.026) at 2 years. However, when corrected for multiple comparisons, this finding was no longer significant. Maternal tertiary education was significantly associated with increased scores in the cognitive domain at 2 years after correction (Exp(B) [95%CI]: 1.94 [0.42 - 3.46], p = 0.013).
Table 5. Multivariable linear regression: adjusted model of the associations between preeclampsia and gestational hypertension compared to normotensive pregnancy exposure versus BSID-III scaled scores
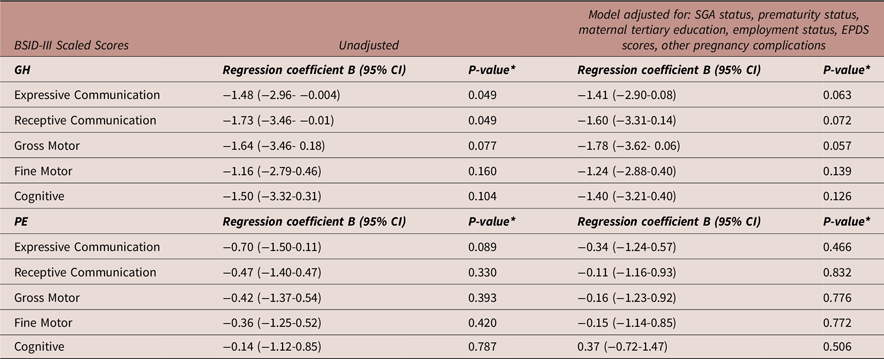
Abbreviations: BSID-III, Bayley Scales of Infant and Toddler Development, 3rd Edition; CI, confidence interval; GH, gestational hypertension; N, number; PE, preeclampsia; SGA, small for gestational age. N = 301 for all models. *A p-value of 0.016 was considered statistically significant (Bonferroni correction).
Table 6 details a binary logistic regression model (same factors adjusted as for the 2-year ASQ-3) exploring the association between PE exposure compared to NTP exposure with the presence of developmental delay at 2-years, as assessed by the BSID-III. PE exposure was not associated with the presence of developmental delay in any domain at 2 years. No regression analysis was performed for the GH exposure group as no infants had scores indicative of developmental delay at 2 years in BSID-III domains (Table 3).
Table 6. Binary logistic regression: adjusted model of the associations between preeclampsia compared to normotensive pregnancy exposure versus presence of developmental delay (BSID-III)
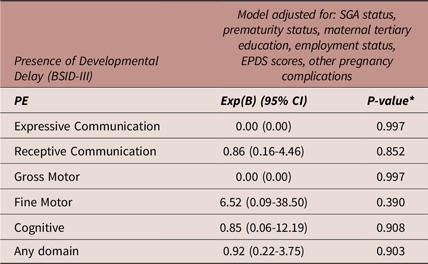
Abbreviations: BSID-III, Bayley Scales of Infant and Toddler Development, 3rd Edition; CI, confidence interval; Exp(B), Exponent beta; N, number; PE, preeclampsia; SGA, small for gestational age. N = 301 for all models. *A p-value of 0.016 was considered statistically significant (Bonferroni correction).
Discussion
In our population of 369 infants, PE-exposed infants were more likely to be born preterm, SGA, be admitted to a NICU or SCN, and breastfed for a shorter duration than infants born after NTP. After adjustment for confounders including SGA and prematurity status, we demonstrated no significant associations of PE or GH exposure with impaired or delayed psychomotor neurodevelopment at 6 months or 2 years. GH was only associated with a slightly increased risk of failing the problem-solving domain of the ASQ-3 screening tool at 2 years before corrections for multiple comparisons. However, this did not correlate to statistically significant differences in BSID-III scaled scores or the proportion of infants with developmental delay in problem-solving or other neurodevelopmental domains. Further, the small sample size of the GH group and the marginal significance of the non-corrected finding indicates a need to interpret this finding with caution.
This is one of the first studies to distinguish between PE and GH exposure when assessing infant neurodevelopment using the BSID-III and ASQ-3 tools. GH has been associated with decreased development quotient on social behaviour at 6 months Reference Chen, Li and Liu19 and increased risk of mild cognitive dysfunction in childhood Reference Heikura, Hartikainen and Nordström20 in children exposed to GH compared to NTP. This may be explained by factors associated with maternal GH such as an adverse genetic cardiometabolic profile, and postnatal lifestyle factors such as maternal socioeconomic status and mental health, associated with negative implications on infant neurodevelopment. Reference Vakil, Henry, Craig and Gow5,Reference Olson, Chen and Fishman21,Reference Rogers, Obst and Teague22 However, after adjustment for common confounders and multiple comparisons, we demonstrated GH was not associated with impaired psychomotor development at 6 months or 2 years on the ASQ-3, a parent-completed screening tool useful for indicating developmental risk but unable to diagnose developmental delay, nor the BSID-III, which is an objective developmental assessment tool used to diagnose developmental delay. Reference Bellman, Byrne and Sege18
PE exposure may have greater potential than GH to impair fetal neurodevelopment. Reference Barker23 Proposed mechanisms associated with PE include early angiogenic imbalances, maternal inflammation, oxidative stress and uteroplacental insufficiency, which may cause fetal hypoxic-ischaemic brain injury and impair later neurodevelopment. Reference de Souza Rugolo, Bentlin and Trindade24 In addition, the Developmental Origins of Health and Disease hypothesis suggests the fetus undergoes ‘developmental programming’ as an adaptation to this adverse intrauterine environment, increasing their future risk of morbidity. Reference Barker23 However, in our cohort neither PE nor GH exposure were associated with poorer BSID-III neurodevelopmental outcomes. Many studies that reported an increased risk of impaired cognitive functioning and neurodevelopment after HDP exposure, Reference Tuovinen, Eriksson, Kajantie and Räikkönen3,Reference Maher, O’Keeffe and Kearney4 and studies that reported no difference in motor, social, mental or cognitive development, Reference Chen, Li and Liu19,Reference Maher, O’Keeffe and O’Keeffe25,Reference Schlapbach, Ersch and Adams26 were conducted in SGA or very preterm (< 32 weeks) populations. PE is associated with fetal growth restriction and subsequent SGA birth, and as delivery is the only definitive treatment for PE, a higher proportion of neonates are born preterm. Reference de Souza Rugolo, Bentlin and Trindade24,Reference Lowe, Bowyer and Lust27 SGA and preterm birth are comorbidities independently associated with poor neurodevelopment, Reference Thomaidis, Zantopoulos and Fouzas6 and subsequently, determining the impact of PE on neurodevelopment independent of these associations is difficult to discern. Importantly, our findings in a mixed cohort of gestations and birthweights, more reflective of the population distribution of HDP, with adjustment for SGA and prematurity status, add to two large prospective cohort studies in mixed populations that reported no significant differences in the proportion of infants with neurodevelopmental delay at 6 months, Reference Chen, Li and Liu19 or developmental risk on the ASQ-3 at 9 months Reference Maher, O’Keeffe and O’Keeffe25 after PE exposure.
In infants exposed to severe PE, one study demonstrated no statistically significant differences in the proportion of infants with failed ASQ domains at 1 and 2 years, but greater failures by 3 years. Reference Warshafsky, Pudwell, Walker, Wen and Smith28 Although assessing neurodevelopmental outcomes later in life may be confounded by lifestyle and behavioural factors, continued follow-up of our cohort will assess whether 2-year trends such as greater proportions of developmental delay in the PE group, and lower BSID-III scaled scores in the GH group, may become statistically and clinically significant as developmental demands increase after infancy toward school age.
HDP exposure and SGA status were not significant predictors in our cohort, however preterm birth was an independent predictor of failing any ASQ-3 domain at 2 years. In preterm infants, PE has been reported as an independent risk factor for neurodevelopmental disability, Reference Johnson, Evans and Draper29 and preterm PE infants had poorer fine motor skills and visuo-auditory perception at 18 months than preterm NTP infants, while term infants had better motor skills, visuo-auditory perception, and social abilities. Reference Martikainen30 HDP are clinically heterogenous, with early onset (< 34 weeks’ gestation) or preterm PE (34–36 + 6 weeks’ gestation), associated with more severe neurodevelopmental impacts than late-onset PE (≥37 weeks), more commonly experienced by our PE cohort. Reference Wang, László and Gissler31,Reference Tranquilli, Brown, Zeeman, Dekker and Sibai32 This may reflect the greater uteroplacental insufficiency associated with early onset or more severe disease, or prolonged fetal exposure to the adverse intrauterine environment where delivery is delayed to avoid preterm birth. Reference de Souza Rugolo, Bentlin and Trindade24,Reference Lowe, Bowyer and Lust27,Reference Tranquilli, Brown, Zeeman, Dekker and Sibai32 However, it is impossible to exclude the influence of other complications of preterm birth including longer NICU or SCN stay and reduced breastfeeding rates at discharge, which are independently associated with impaired neurodevelopment. Reference Johnson, Evans and Draper29 While PE was not associated with adverse neurodevelopmental outcomes in our cohort, we were unable to stratify PE exposure by onset or severity due to sample size. Subsequently, further studies with adjustment for prematurity and SGA status are indicated to further elucidate any independent impact of severe HDP exposure on infant neurodevelopment.
Strengths of this study include our recruitment of an ethnically diverse and mixed cohort of infants born at varying gestations and birthweights, with control for several known and proposed determinants of child neurodevelopment including preterm birth, SGA status, maternal education and employment, maternal postnatal mental health, and other maternal complications including gestational diabetes mellitus. Reference Rogers, Obst and Teague22,Reference Donald, Wedderburn and Barnett33 The validated developmental screening tool, the ASQ-3, is useful in primary care and general paediatric settings as it is readily available, quick to complete and parent-completed. Reference Vitrikas, Savard and Bucaj34 The BSID-III is a gold-standard developmental assessment tool for diagnosis of developmental delay. Reference Stein, Lukasik, Carey, Crocker, Coleman, Elias and Feldman35 Prior studies assessing the association of HDP with infant neurodevelopment have usually considered assessment and screening scores only, rather than clinically significant thresholds indicating future childhood cognitive and motor performance. Subsequently, our analyses of infants with failed ASQ-3 domains and developmental delay in BSID-III domains adds to the few studies assessing HDP and infant neurodevelopment with clinically significant outcomes rather than scores, using readily available and validated tools. However, we must note that the population from which the BSID-III was derived from included children with varied clinical conditions that may impact neurodevelopment, such as prenatal alcohol exposure and premature birth. Thus, the tool may underestimate the presence of delay, and not identify children with mild to moderate delay. Reference Piñon, Weiss, Oakland and Aylward36,Reference Duggan, Irvine, O’B Hourihane, Kiely and Murray37 This may explain why on univariate analyses, GH-exposed infants had on average lower BSID-III scaled scores in all domains compared to NTP infants, however these scores did not cross the threshold for developmental delay in any domain.
Limitations include the single-centre nature of the P4 study, and that it was powered to detect differences in maternal rather than paediatric health outcomes specifically. The small sample size of GH-exposed infants reduced statistical power, and the moderately sized PE group prevented sub-analyses based on PE onset or severity. Although our final cohort was ethnically diverse, participants were excluded if they did not have a good understanding of written and spoken English, limiting the applicability of our findings to culturally and linguistically diverse populations. We also lacked data on other confounding factors that may influence infant development, including infant hospitalisations and comorbidities, parental prenatal mental health, paternal employment, and maternal alcohol consumption during pregnancy itself, despite no significant differences in maternal alcohol consumption between groups at 6 months and 2 years (Table 1). A high proportion of parents in our cohort completed tertiary education and we demonstrated that maternal tertiary education is associated with increased scores in the cognitive domain at 2 years. Parental education is an indicator of higher socioeconomic status, directly associated with early childhood neurodevelopment. Reference Olson, Chen and Fishman21 So, while HDP may not influence infant neurodevelopment in high income populations, our findings may not be generalisable to populations of lower socioeconomic status. Due to high-level, consistent HDP care in the study hospital, HDP-exposed mothers and children had additional antenatal and postnatal health visits and study participants likely had greater health awareness than the general population. Subsequently we cannot exclude the influence of healthy volunteer bias in our sample masking a negative influence of HDP on infant neurodevelopment.
Our findings are broadly reassuring regarding infant neurodevelopment after HDP, especially in populations with protective sociodemographic and clinical care factors. The comorbid complication of preterm birth in the PE group was associated with poorer neurodevelopment, highlighting the need for greater parental and physician awareness and early surveillance of neurodevelopment in HDP-exposed, premature infants. Early intervention may alter a child’s neurodevelopmental trajectory before school age, where delay may confer more severe cognitive, psychosocial and behavioural consequences. Reference Doyle, Harmon, Heckman and Tremblay38 Our research also highlights opportunities to optimise breastfeeding duration in hypertensive groups, as breastfeeding is positively associated with cognitive development, Reference Bar, Milanaik and Adesman39 and promote key socioeconomic determinants of health like parental education and preschool attendance at individual, community and policy levels to optimise paediatric neurodevelopmental outcomes.
We demonstrated no strong association of PE or GH exposure, but a strong association of prematurity status, with abnormal psychomotor developmental outcomes in infants at 6 months or 2 years. Although reassuring, continued follow-up and further research with larger samples and more term-born, non-SGA and socioeconomically diverse cohorts is necessary to elucidate the impacts of GH and PE exposure.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2040174425000121.
Acknowledgements
The authors wish to thank psychologists Carly Black, Andrew Webb, Rachel Rohde, Taryn Hayhurst, Esther Chan, Diana Grujaska, Julie Co., Lauren Dearlove, and James McIntosh for conducting the BSID-III assessments, the families for their ongoing participation in the P4 study, the St George Obstetric Medicine Research Group and prior UNSW medical students for their assistance with data collection and providing administration for the P4 study.
Financial support
This cohort study was supported by the St George and Sutherland Medical Research Foundation and Emeritus Professor Richard Henry (philanthropic donation), and authors received funding from the National Health and Medical Research Council (S.W., grant number APP1158954, and M.G., grant number APP1158876).
Competing interests
The authors declare none.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the Australian National Statement on Ethical Conduct in Human Research of 2007, and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the South-Eastern Sydney Local Health District Human Research Ethics Committee (HREC/12/POWH395).










