Introduction
The intertidal shoreline is a zone of high biodiversity that supports a rich fauna of invertebrates, fish and shorebirds (Sokolova, Reference Sokolova1995). As parasites that use different host species sequentially throughout their complex life cycle, the diversity of trematodes in molluscs (first intermediate host) appears to depend on the diversity of benthic invertebrates and fish (second intermediate host) and that of vertebrates, mainly birds and fish (definitive host) (Bustnes & Galaktionov, Reference Bustnes and Galaktionov1999; Smith, Reference Smith2001; Hechinger & Lafferty, Reference Hechinger and Lafferty2005; Huspeni et al., Reference Huspeni, Hechinger, Lafferty and Bortones2005). Trematode infection in intertidal gastropods can be determined by large-scale factors (climate, occurrence of hosts in the geographical region) and small-scale factors (tidal level, substrate type, distribution of intermediate and definitive hosts in the local habitat, host abundance) (Skirnisson & Galaktionov, Reference Skirnisson and Galaktionov2002; Poulin & Mouritsen, Reference Poulin and Mouritsen2003). However, some studies have indicated that the latter may be more important than the former in determining the number of trematode species infecting snails, and the proportion of infected snails in a population (Combes, Reference Combes2001, Poulin & Mouritsen, Reference Poulin and Mouritsen2003). Generally, the abundance of shorebirds plays a crucial role and has been found to be directly related to digenean diversity (Hechinger & Lafferty, Reference Hechinger and Lafferty2005; Fredensborg et al., Reference Fredensborg, Mouritsen and Poulin2006).
The taxonomic identification of larval digeneans is a key step towards the elucidation of their life cycles, which facilitates increased understanding of biodiversity and the use of these parasites as a predictive tool in ecological studies (Nolan & Cribb, Reference Nolan and Cribb2005). Knowledge of marine cercariae in the south-western Atlantic Ocean has increased in recent years (e.g. Averbuj & Cremont, Reference Averbuj and Cremonte2010; Gilardoni et al., Reference Gilardoni2011; Bagnato et al., Reference Bagnato2015). Seven digenean species were recorded parasitizing four gastropod species in Puerto Madryn (42°S, 64oW), a northern site on the Patagonian coast of Argentina (Gilardoni et al., Reference Gilardoni2011). To date, larvae of 31 species of marine digeneans from gastropods and bivalves have been recorded on the Argentine coasts by Bagnato et al. (Reference Bagnato2015), including those from Puerto Deseado (47°S, 65°W), many of which remain undescribed.
The goal of this study is to describe the larval digeneans that parasitize gastropods from Puerto Deseado, Argentina (recorded in Bagnato et al., Reference Bagnato2015) using morphological and molecular data. An additional goal is to compare the parasite diversity at this locality with that at a more northern site, Puerto Madryn, using the data provided by Gilardoni et al. (Reference Gilardoni2011).
Materials and methods
A total of 1758 specimens of seven gastropod species (266 Crepipatella dilatata, 151 Fissurella radiosa, 306 Nacella magellanica, 458 Pareuthria fuscata, 237 Siphonaria lateralis, 186 S. lessonii and 154 Trophon geversianus) were collected from the rocky littoral zone near the mouth of the estuary of the Deseado River (47°45′S, 65°55′W), at Puerto Deseado, Santa Cruz Province, Argentina, south-western Atlantic Ocean. Host names are used in accordance with the World Register of Marine Species (WoRMS, 2017). During the lowest tides, in July 2009 (austral winter) and February 2010 (austral summer), specimens of each species were collected at random, by hand, from throughout their intertidal levels, following transects perpendicular to the coast in a 200 m2 area. All parasite species were recorded but only new species were described. The gastropods were transported live to the laboratory and placed in small flasks filled with seawater at room temperature (20–23°C), and were inspected twice daily under a stereomicroscope for emerged cercariae. The latter were stained with vital stain (neutral red or Nile blue) and studied alive under a light microscope before being fixed. After 48 hours all gastropods were necropsied to detect prepatent infections and to examine parthenogenetic generations such as sporocysts or rediae, and metacercariae. Several naturally emerged cercariae, parthenites and metacercariae (c. 50 specimens of each stage) were killed with warm seawater, fixed immediately with 10% formalin (Cribb & Bray, Reference Cribb and Bray2010), stained with Semichon's acetocarmine or Gomori's trichrome, dehydrated through ascending ethanol series, cleared with methyl salicylate, and mounted on glass slides with Canada balsam. Drawings and measurements were obtained for 15–20 unflattened, fixed, stained and mounted specimens, with the aid of a light microscope with a drawing device. Measurements are presented as mean values in micrometers, followed by the range in parentheses. The forebody of cercariae was measured as the distance from the anterior end to the anterior edge of the ventral sucker. Prevalences were calculated following Bush et al. (Reference Bush1997). For scanning electron microscopy (SEM), some specimens were fixed in a 2.5% glutaraldehyde solution buffered with 0.1 M sodium cacodylate, dehydrated through an ascending ethanol series and dried by rinsing for a few minutes in hexamethyldisilazane. Photomicrographs were obtained with a JSM-6460LV SEM (JEOL Ltd, Akishima, Japan) operating at 15 kV. Mounted specimens of new species were deposited at the Parasitological Collection (CNP-Par) of Instituto de Biología de Organismos Marinos (IBIOMAR), Puerto Madryn, Argentina. Molecular data were taken from Bagnato et al. (Reference Bagnato2015) for species described for the first time. For each studied species, comparisons with other available sequences of the same family were made using NCBI BLAST (https://blast.ncbi.nlm.nih.gov/) to support the morphological diagnosis. A microphallid species parasitizing C. dilatata was previously described by Gilardoni et al. (Reference Gilardoni2011) and erroneously identified as Maritrema sp. 1; however, comparison of molecular data with NCBI BLAST indicated that the sequence showed low similarity with other species of the genus Maritrema (69–77% in ITS1 and 83–86% in ITS2). For this reason, the larva is renamed as Microphallidae gen. et sp.1 until the taxonomic identity and life cycle can be established. Larval digeneans parasitizing the most common gastropods in the northern site of Puerto Madryn, Chubut Province (42°46′S, 64°54′W) were recorded by Gilardoni et al. (Reference Gilardoni2011). Analyses of diversity measures were performed considering only samplings carried out in winter 2009 and summer 2010 in Puerto Madryn (n = 710) and Puerto Deseado; both samplings were identical and only the parasites that use gastropods as first intermediate host were included (i.e. Gymnophalloides nacellae was excluded because it occurred as metacercaria). For both sites, species richness (Sg) of parasitized gastropods, and species richness (Sp), Shannon–Wiener diversity (H´) and Shannon equitability (E) indices were calculated (Magurran, Reference Magurran2004). For both gastropods and parasites, species richness was calculated as the number of species present at each site. To calculate the H´ index, prevalence (p i) (considered an abundance measure) was calculated as the number of gastropods of species X infected by a parasite species Y divided by the total number of gastropods of species X examined. The H´ index was calculated as ![]() $\; \mathop \sum p_{i}lnp_{i} $. Additionally, the H´ index was calculated considering only parasite species found to occur at both sites. The diversity indices were compared by the Hutcheson method associated with variance of H´ (Hutcheson, Reference Hutcheson1970). The Shannon equitability index is a measure of heterogeneity and is calculated as the ratio of the observed diversity to the maximum diversity (
$\; \mathop \sum p_{i}lnp_{i} $. Additionally, the H´ index was calculated considering only parasite species found to occur at both sites. The diversity indices were compared by the Hutcheson method associated with variance of H´ (Hutcheson, Reference Hutcheson1970). The Shannon equitability index is a measure of heterogeneity and is calculated as the ratio of the observed diversity to the maximum diversity (![]() $H^{\prime}/H_{max}$ =
$H^{\prime}/H_{max}$ = ![]() $H^{\prime} / ln S,\; {\rm where\; S\; is\; the\; species\; richness}$). To evaluate the dominance of a given species at each site studied, the Simpson dominance index (D) was calculated as Σpi2. To compare the similarity between parasite communities at both study sites, the Jaccard index (Ij) was calculated (equation 1) (Magurran, Reference Magurran2004) as follows:
$H^{\prime} / ln S,\; {\rm where\; S\; is\; the\; species\; richness}$). To evaluate the dominance of a given species at each site studied, the Simpson dominance index (D) was calculated as Σpi2. To compare the similarity between parasite communities at both study sites, the Jaccard index (Ij) was calculated (equation 1) (Magurran, Reference Magurran2004) as follows:
where a is the number of species present only at site A, b is the number of species present only at site B, and c is the number of species present at both sites (A and B).
Results
Parasite survey and description of new larval forms
Twelve species of digenean trematodes belonging to nine families were found parasitizing six out of the seven surveyed gastropod species. No parasite was found in Fissurella radiosa (n = 151). All parasite species and their prevalence are listed in table 1; five of them are new larvae and are here described.
Table 1. Prevalence and diversity indexes of digenean larvae from gastropod species in Puerto Deseado, Santa Cruz Province and Puerto Madryn, Chubut Province, Argentina, during winter 2009 and summer 2010 (Gymnophalloides nacellae is excluded because it was found only at the metacercaria stage). Parasite species in bold are described in the present study; all species were recorded previously by Gilardoni et al. (Reference Gilardoni2011) and Bagnato et al. (Reference Bagnato2015).

aAbsent, gastropod and parasite absent at the site; No (not observed), gastropod and parasite are present at the site but were not observed in the samples analysed.
Family Lepocreadiidae Odhner, 1905
Lepocreadiidae gen. et sp. 1
Redia. Body elongate, posterior end pointed, 669 (491–829) long by 130 (105–154) maximum wide, wall 5 (3–7) thick. Birth pore anterior and lateral. Pharynx large, 70 (50–82) long by 50 (43–64) wide; intestine observed in young rediae, masked by cercariae in fully mature rediae. Usually 15 (5–29) larvae per redia at different development stages, 12 (5–29) cercariae and 3 (2–6) germinal balls. The latter mainly located posteriorly; cercariae increase in size and complete development outside of rediae.
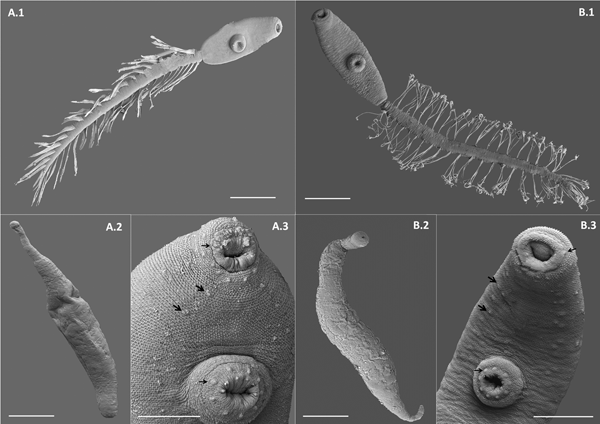
Cercaria. Ophthalmotrichocercous. Body spinous, 226 (145–286) long by 98 (84–125) wide at level of ventral sucker. Two lateral rows of eight papillae from posterior end of oral sucker to posterior end of ventral sucker (seen only with SEM, fig. 3A.3). One pair of eyespots, oval-shaped, darkly pigmented (fig. 2A), at pharynx level, 24 (17–31) long by 17 (14–20) wide. Oral sucker 46 (34–54) long by 53 (38–72) wide; mouth subterminal, surrounded by numerous papillae; at least 12 at anterior half of oral sucker; an outer circle of at least eight papillae and an inner circle of at least four papillae (seen only with SEM, fig. 3A.3). Forebody 106 (78–109) long. Prepharynx 20 (14–31) long, pharynx muscular 21 (15–24) long by 18 (13–25) wide, oesophagus short 16 (11–21) long, bifurcating anteriorly to ventral sucker. Caeca narrow 153 (93–174) long, extending to posterior end of body. Ventral sucker at mid-level of body 39 (29–48) long by 45 (41–49) wide, with an outer circle of nine papillae and an inner circle of six papillae at anterior half (seen only with SEM, fig. 3A.3). Sucker ratio 1 : 0.98 (0.91–1.2). Four pairs of penetration glands opening dorsally at anterior end of body; none of these glands stained with neutral red; cytons located posteriorly of eyespots at oesophagus level (fig. 2A). Ovary pretesticular 11 (8–15) long by 16 (10–21) wide, posterior to ventral sucker; two testes in tandem, anterior testis 12 (8–17) long by 22 (15–27) wide and posterior testis 14 (9–19) long by 23 (19–29) wide. Metraterm and cirrus sac opening in a genital atrium located anteriorly and at right of ventral sucker (fig. 1A.4). Excretory vesicle elongate and narrow, 145 (103–180) long by 14 (5–31) wide, width variable depending on degree of repletion; extending and bending around ventral sucker, filled with large and refractive excretion granules (fig. 2A). Limbs from excretory vesicle branch at cytons level. Muscular sphincter at posterior end of excretory vesicle. Excretory formula not determined, only some flame cells observed. Tail long (more than twice length of body), 566 (435–692) long by 35 (28–39) wide at base, with 25 pairs of setae groups, setae 107 (97–120) long, c. five to seven setae per group, and a pair in tip of tail.
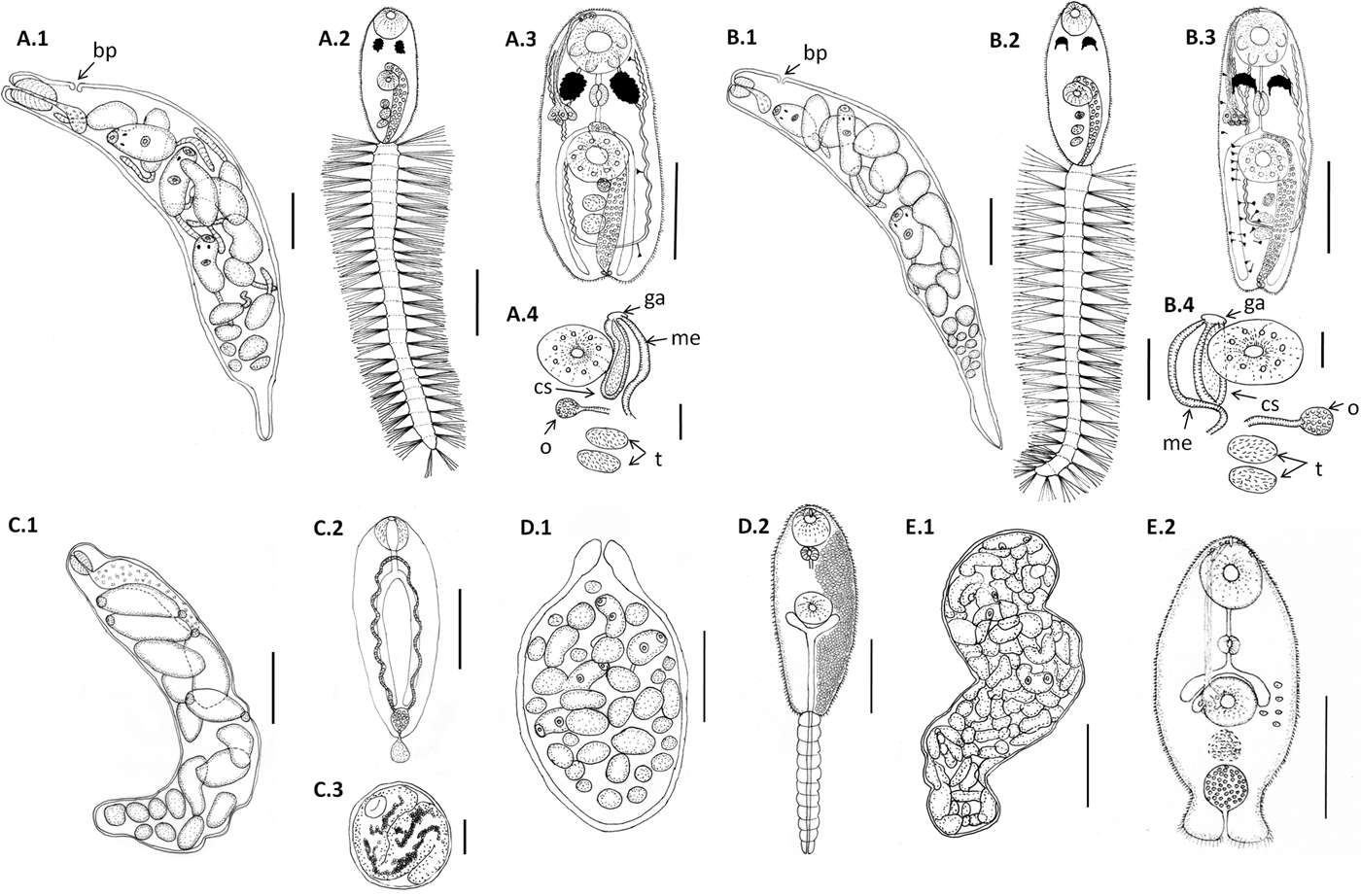
Fig. 1. Line drawings of larval digeneans described in this study that parasitize snails from Puerto Deseado, Argentina. (A) Lepocreadiidae gen. et sp. 1: (A.1) redia; (A.2) cercaria, ventral view; (A.3) body of cercaria, ventral view, penetration glands on left side and flame cells on right side omitted; (A.4) detail of genitalia. (B) Lepocreadiidae gen. et sp. 2: (B.1) redia; (B.2) cercaria, ventral view; (B.3) body of cercaria, ventral view, penetration glands and flame cells on left side omitted; (B.4) detail of genitalia. (C) Notocotylidae gen. et sp. 1: (C.1) rediae; (C.2) cercaria, dorsal view; (C.3) encysted metacercaria from inside the redia. (D) Renicolidae gen. et sp. 2: (D.1) sporocyst; (D.2) cercaria, ventral view, cystogen cells on right side omitted. (E) Zoogonidae gen. et sp. 1: (E.1) sporocyst; (E.2) cercaria, ventral view, penetration glands on left side omitted. Abbreviations: ga, genital atrium; me, metraterm; o, ovary; cs, cirrus sac; t, testis. Scale bars: (C.1, E.1) 200 μm; (A.1, A.2, A.3, B.1, B.2, B.3, C.2, D.1, D.2) 100 μm; (E.2) 50 μm; (A.4, B.4) 20 μm.
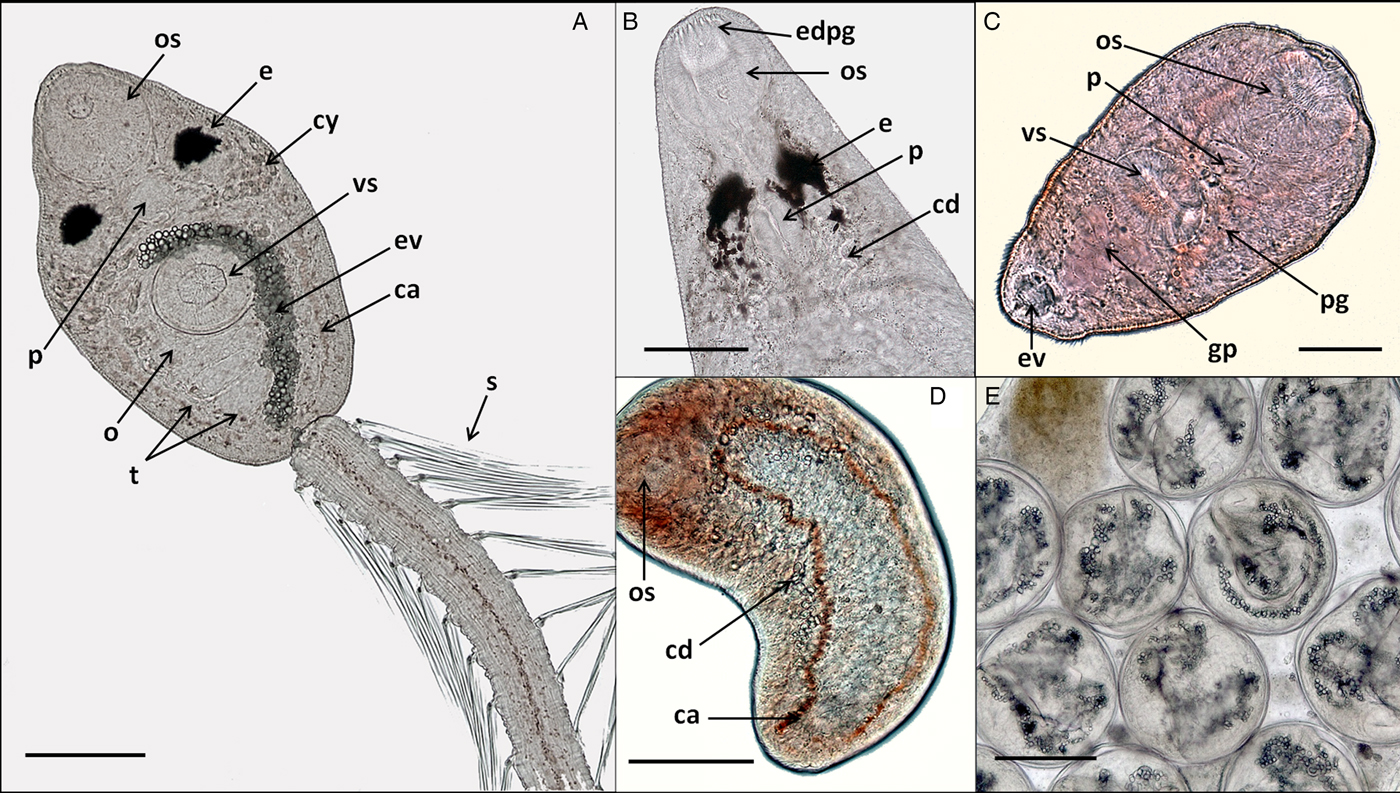
Fig. 2. In vivo light microscope photographs of lepocreadiid, notocotylid and zoogonid species from Puerto Deseado, Argentina. (A) Cercaria of Lepocreadiidae gen. et sp. 1, ventral view; (B) cercaria (anterior part) of Lepocreadiidae gen. et sp. 2, dorsal view; (C) cercaria of Zoogonidae gen. et sp. 1, ventral view; (D) cercaria of Notocotylidae gen. et sp. 1, dorsal view, collector ducts joining caeca at the oesophagus level; (E) metacercaria of Notocotylidae gen. et sp. 1 inside the redia. Abbreviations: ca, caeca; cd, collector ducts; cy, cytons; e, eyespot; edpg, end ducts of penetration glands; ev, excretory vesicle; gp, genital primordium; o, ovary; os, oral sucker; p, pharynx; pg, penetration glands; t, testis; s, spines; vs, ventral sucker. Scale bars: (A, E) 100 μm; (B, D) 50 μm; (C) 25 μm.

Fig. 3. Scanning electron microscope (SEM) photographs of two lepocreadiid species from Puerto Deseado, Argentina. (A) Lepocreadiidae gen. et sp. 1: (A.1) cercaria, ventral view; (A.2) redia; (A.3) detail of anterior part, ventral view, papillae in oral sucker, in ventral sucker (arrows) and on the body along two longitudinal rows (head arrows). (B) Lepocreadiidae gen. et sp. 2: (B.1) cercaria, ventral view; (B.2) redia; (B.3) detail of anterior part, ventral view, papillae in oral sucker, in ventral sucker (arrows) and on the body along two longitudinal rows (head arrows). Scale bars: (A.1, A.2, B.1, B.2) 100 μm; (A.3, B.3) 50 μm.
Taxonomic summary
Host. Crepipatella dilatata (Lamarck) (Caenogastropoda, Calyptraeidae).
Infection site. Gonad and digestive gland.
Locality. Puerto Deseado (47°45′S, 65°55′W), Santa Cruz Province, Argentina.
Deposited material. CNP-Par 52.
GenBank accession numbers. KF451932 (18S and partial ITS1), KF451933 (ITS2 and 28S).
Remarks
The following morphological features indicate that the larvae here described belong to the family Lepocreadiidae according to Cable (Reference Cable1963): distome, eyespots present, trichocercous, development inside rediae, spiny body, papillae present, and ornamented tail with pairs of lateral setae (individual or in groups), which are not joined by a membrane forming fins. Intestinal caeca extending to the posterior end of the body. Excretory system variable and saccular vesicle extending to the ventral sucker. To date, 23 species of cercariae belonging to this family have been described (Villot, Reference Villot1875; Macfarlane, Reference Macfarlane1951; Ito & Shimura, Reference Ito and Shimura1980; Stunkard Reference Stunkard1980a, Reference Stunkardb; Watson, Reference Watson1984; Aguirre-Macedo & Kennedy, Reference Aguirre-Macedo and Kennedy1999; Hassanine, Reference Hassanine2006; Averbuj & Cremonte, Reference Averbuj and Cremonte2010 and references therein). Six of them were recorded in the Southern Hemisphere and three were recorded in the Argentinean Sea, including the cercariae here described (Martorelli, Reference Martorelli1991; Averbuj & Cremonte, Reference Averbuj and Cremonte2010; Bagnato et al., Reference Bagnato2015). Lepocreadiidae gen. et sp. 1 is morphologically similar to the cercariae belonging to the genus Lepocreadium Stossich, 1904; it presents four pairs of penetration glands as observed in L. areolatum (Linton, 1900), and the shape of eyespots and the excretory vesicle, and the number of setae of the tail (c. 25 pairs) are similar to those in L. pegorchis (Stossich, 1900) (Bartoli, Reference Bartoli1967; Stunkard, Reference Stunkard1980a).
Lepocreadiidae gen. et sp. 2
Redia. Body elongate, posterior end pointed, 978 (846–1153) long by 172 (143–212) maximum wide, wall 7 (5–8) thick. Birth pore anterior and lateral. Pharynx large, 48 (40–59) in diameter, intestine observed in young rediae, masked by cercariae in fully mature rediae. Usually 25 (11–34) larvae per redia at different development stages, 16 (9–23) cercariae and 9 (3–15) germinal balls. The latter mainly located posteriorly, cercariae increase in size and complete development outside of redia.
Cercaria. Ophthalmotrichocercous. Body spinous, 308 (219–389) long by 94 (76–132) wide at level of ventral sucker. Two lateral rows of eight papillae from posterior end of oral sucker to posterior end of ventral sucker (seen only with SEM, fig. 3B.3). A pair of eyespots half-moon shaped, darkly pigmented (fig. 2B), anterior to the pharynx level, 22 (16–32) long by 14 (8–24) wide. Oral sucker 58 (51–63) long by 56 (45–68) wide, mouth subterminal, surrounded by numerous papillae: at least 12 at anterior half of oral sucker and four larger papillae at posterior half of ventral sucker (seen only with SEM, fig. 3B.3). Forebody 154 (126–185) long. Prepharynx 27 (17–44) long; pharynx muscular, 27 (16–35) long by 18 (15–21) wide; oesophagus short, 13 (9–19) long, bifurcating immediately anterior to ventral sucker. Caeca narrow, 177 (139–219) long, extending to posterior end of body. Ventral sucker at mid-level of body, 48 (39–54) long by 46 (38–58) wide, with an outer circle of nine papillae and an inner circle of six papillae at anterior half (seen only with SEM, fig. 3B.3). Sucker ratio 1–0.86 (0.8–0.87). Six pairs of penetration glands opening dorsally at anterior end of body (fig. 2B), none of which stained with neutral red; cytons located posteriorly to eyespots at oesophagus level. Ovary pretesticular, 15 (9–24) long by 15 (9–24) wide, posterior to ventral sucker; two testes in tandem, anterior testis 17 (11–22) long by 21 (14–28) wide and posterior testis 18 (14–23) long by 22 (15–29) wide. Metraterm and cirrus sac opening in a genital atrium located anteriorly and at left of ventral sucker (fig. 1B.4). Excretory vesicle elongate and narrow, 183 (123–252) long by 34 (14–46) wide, width variable depending on degree of repletion, extending and bending around ventral sucker, filled with large and refractive excretion granules. Limbs from excretory vesicle branch at cytons level. Muscular sphincter at posterior end of excretory vesicle. Excretory formula not determined, only some flame cells observed. Tail long (more than twice length of body), 662 (424–951) long by 34 (30–42) wide at base, with 23 groups of setae per side 102 (81–123) long, c. five to seven setae per group, and a pair in tip of tail.
Taxonomic summary
Host. Pareuthria fuscata (Bruguière) (= Pareuthria plumbea (Philippi)) (Caenogastropoda, Buccinidae).
Infection site. Gonad and digestive gland.
Locality. Puerto Deseado (47°45′S, 65°55′W), Santa Cruz Province, Argentina.
Deposited material. CNP-Par 53.
GenBank accession numbers. KF451934 (18S and partial ITS1), KF451935 (ITS2 and 28S).
Remarks
The cercaria of Lepocreadiidae gen. et sp. 2 is similar to the cercariae belonging to the genus Opechona Looss, 1907. It exhibits half-moon shaped eyespots and 23 pairs of setae groups in the tail, comparable to those of O. pyriforme (Linton, 1900) and O. cablei cercariae (Stunkard, Reference Stunkard1969b, Reference Stunkard1980b, Reference Stunkard1983). Cercariae of Lepocreadiidae gen. et sp. 1 (from Crepipatella dilatata) differ from those of Lepocreadiidae gen. et sp. 2 (from Pareuthria fuscata) in the number of penetration glands (four vs six) and in the shape of eyespots (oval vs half-moon shaped). Molecular results show high similarity (~99%) between both Lepocreadiidae species here described, species of the genus Opechona such as Opechona sp. (KF451938.1), O. kahawai Bray & Cribb, 2003 (FJ788491.1), and Lepocreadium album (Stossich, 1890) (KF656703.1 and KF656704.1) (the only sequenced species of Lepocreadium) (Palombi, Reference Palombi1937; Russell-Pinto et al., Reference Russell-Pinto, Gonçalves and Bowers2006). Members of the genera Lepocreadium and Opechona share several morphological characters, including size, eyespots, excretory vesicle, and setae in the tail (Stunkard, Reference Stunkard1969a). Ribosomal DNA sequences of several genera of the Lepocreadiidae family are highly similar (between 81 and 99%). It is necessary to identify these larvae at the genus and species level to find the adults from coastal fish. The morphological and molecular differences (between 0.3 and 1.66% among sequences) between the two larval lepocreadiids here described, and the fact that both species parasitize different snails support the existence of two different species.
Notocotylidae Lühe, 1909
Notocotylidae gen. et sp. 1
Redia. Only one snail was found parasitized and most rediae were full with metacercariae (fig. 2E) and could not be measured because they were broken. Measurements were taken from rediae with cercariae (n = 15). Body large and elongate, 950 (700–1171) long by 212 (166–276) maximum wide, wall 10 (6–13) thick. Birth pore not observed. Pharynx 60 (38–74) in diameter; intestinal caeca saccular, 224 (172–274) long by 53 (36–82) wide. Usually 16 (8–22) larvae per redia at different development stages, 11 (4–17) cercariae and 6 (3–9) germinal balls.
Cercaria. Monostome. Body small and oval, 299 (229–494) long by 97 (69–161) wide. Oral sucker 45 (34–66) long by 47 (37–75) wide; mouth terminal. Prepharynx and pharynx absent. Oesophagus short, 34 (27–47) long; caeca long, 184 (165–217) extending to posterior end of body. Excretory vesicle small and oval, 41 (21–65) long by 36 (15–50) wide; collector ducts long, extending parallel to caeca up to the oesophagus level, where they join (fig. 2D). Tail small and rounded, 34 (25–43) long by 29 (25–33) wide.
Metacercaria. Cyst spherical, 168 (128–190) in diameter. Wall 5 (4–8) thick. Oral sucker 43 (35–51) in diameter; excretory vesicle well developed, with large and refractive excretion granules (fig. 2E).
Taxonomic summary
Host. Nacella magellanica (Gmelin) (Patellogastropoda, Nacellidae).
Infection site. Gonad and digestive gland.
Locality. Puerto Deseado (47°45′S, 65°55′W), Santa Cruz Province, Argentina.
Deposited material. CNP-Par 57.
GenBank accession number. KF656705 (ITS1).
Remarks
The following morphological features indicate that the larvae here described belong to the family Notocotylidae according to Rotschild (Reference Rotschild1938): monostome; pharynx absent; small and oval excretory vesicle with two collector ducts extending to the oesophagus level, where they join, forming a close tubular junction; penetration glands absent (Rotschild, Reference Rotschild1938; Yamaguti, Reference Yamaguti1975). There are two groups of notocotylid cercariae: (1) cercariae with eyespots and a long tail, and (2) cercariae without eyespots and with a short tail or without a tail. The cercaria described in the present study belong to the second group, along with Parapronocephalum symmetricum Belopolskaia, 1952, Notocotyloides petasatus (Deslongchamps, 1824), Catatropis verrucosa (Froelich, 1789) Odhner, 1905 and pronocephaloid sp. I (Yamaguti, Reference Yamaguti1975; Hechinger, Reference Hechinger2012). Cercariae of P. symmetricum and N. petasatus were originally located in the family Pronocephalidae Looss, 1899 and were subsequently relocated to the family Notocotylidae (Barton & Blair, Reference Barton, Blair, Jones, Bray and Gibson2005). The cercaria here described was previously recorded parasitizing Nacella deaurata from Patagonia, Argentina as a pronocephalid (Martorelli et al., Reference Martorelli, Morriconi and Marcotegui2005). All mentioned cercariae are similar in the length of caeca and the shape of the excretory vesicle. The cercaria here described is similar to C. verrucosa in body size (c. 300 μm) and in the shape of the metacercaria. In most life cycles of notocotylids belonging to the second group, the metacercaria encysts inside the redia in the first intermediate host, indicating an abbreviated cycle (Yamaguti, Reference Yamaguti1975; Hechinger, Reference Hechinger2012). Molecular results show a high similarity between the sequences of the cercaria here described and those reported from Notocotylus malhamensis (JQ766940.1) (90% in ITS1) and Tristriata anatis (KX833023.1) (93% in ITS1), the only two ITS1 sequences available for the Notocotylidae family. Other sequences (18S, ITS2, 28S) are available for species of this family in GenBank. Future sequencing of these regions could contribute to determining the taxonomic identity of the notocotylid here described.
Renicolidae Dollfus, 1939
Renicolidae gen. et sp. 2
Sporocyst. Body oval, anterior end narrow, 294 (156–488) long by 171 (109–269) wide, wall 11 (8–17) thick. Birth pore terminal. Usually 35 (22–47) larvae per sporocyst at different development stages, 8 (3–22) cercariae and 30 (16–37) germinal balls.
Cercaria. (Description based on not-fully developed specimens; sporocysts with developed cercariae were not found). Xiphidocercaria, distome. Body small and elongate, 154 (120–202) long by 67 (58–75) wide. Cystogenous glands filling the parenchyma from level of pharynx to posterior end of body. Oral sucker 34 (28–42) long by 37 (35–40) wide, mouth subterminal. Forebody 65 (53–84) long. Stylet small and lanceolate. Penetration glands not observed. Prepharynx absent, pharynx 11 (10–12) long by 10 (9–11) wide, oesophagus and caeca not observed. Ventral sucker 31 (28–33) long by 30 (24–39) wide. Sucker ratio 1 : 0.81 (0.68–0.97). Excretory vesicle Y-shaped with long stem and short arms reaching mid-level of ventral sucker, opening at tip of tail. Excretory formula not determined. Tail 128 (117–138) long by 18 (16–23) wide.
Taxonomic summary
Host. Nacella magellanica (Gmelin) (Patellogastropoda, Nacellidae).
Infection site. Digestive gland.
Locality. Puerto Deseado (47°45′S, 65°55′W), Santa Cruz Province, Argentina.
Deposited material. CNP-Par 55.
GenBank accession number. KF358775 (ITS1).
Remarks
The following morphological features define the cercariae here described as belonging to the Renicolidae family: xiphidocercaria, Y-shaped excretory vesicle, prepharynx absent and cystogenous glands numerous. At least 24 renicolid cercariae have been described worldwide (e.g. Gilardoni et al., Reference Gilardoni2011, and references therein). The cercaria here described was recorded previously by Martorelli et al. (Reference Martorelli, Morriconi and Marcotegui2005) parasitizing N. magellanica at a southern locality on the Patagonian coast, Argentina. This is the second report of a renicolid larva from South America (Gilardoni et al., Reference Gilardoni2011; Bagnato et al., Reference Bagnato2015). It is similar to Renicolidae gen. et sp. 1 (from T. geversianus) in the shape of the excretory vesicle and in body size, tail and suckers (Gilardoni et al., Reference Gilardoni2011); however, the percentage of similarity between both sequences is low (73% in ITS1), indicating the existence of two distinct species. No other ITS1 sequence of renicolid is available, but other sequences (18S, ITS2, 28S) are available for species of this family in GenBank. Future sequencing of these regions could help to determine the taxonomic identity of the renicolid here described.
Family Zoogonidae Odhner, 1902
Zoogonidae gen. et sp. 1
Sporocyst. Only one snail parasitized by this species was found, and most cercariae were not fully developed. Body whitish, variable shape (oval, kidney-shaped or S-shaped), 1273 (851–1739) long by 310 (201–421) wide, wall 7 (4–12) thick. Birth pore not observed. Usually 114 (83–147) larvae per sporocyst at different development stages, 37 (10–131) cercariae and 77 (16–125) germinal balls.
Cercaria. (Description based on ten not-fully developed cercariae from sporocysts). Xiphidocercaria, body small and elongate, 124 (106–167) long by 59 (55–66) wide, covered with tiny spines from oral sucker level to posterior end of body. Cystogen cells scattered in parenchyma. Forebody 62 (44–87) long. Oral sucker 30 (24–35) long by 28 (24–33) wide, mouth subterminal and tiny stylet. Prepharynx 18 (15–25) long; pharynx globular, 11 (10–14) long by 12 (10–14) wide; oesophagus short, 12 (8–15) long; and caeca saccular, 16 (12–19) long, extending to mid-ventral sucker. Ventral sucker 24 (22–27) long by 25 (21–30) wide. Sucker ratio 1 : 0.89 (0.87–0.91). Four pairs of penetration glands opening ventrally at stylet base, stained with neutral red; cytons located at ventral sucker level. Excretory vesicle small and oval, 30 (21–35) long by 24 (15–34) wide, filled with excretion granules. Genital primordium 15 (10–21) long by 16 (12–19) wide, located anteriorly to excretory vesicle (fig. 2C). Minute extension of posterior end of body with spines; excretory pore at distal end of the extension.
Taxonomic summary
Host. Pareuthria fuscata (Philippi) (Caenogastropoda, Buccinidae).
Infection site. Gonad and digestive gland.
Locality. Puerto Deseado (47°45′S, 65°55′W), Santa Cruz Province, Argentina.
Deposited material. CNP-Par 54.
GenBank accession number. KF358773 (ITS1, 5.8S and ITS2).
Remarks
The genera Zoogonus Loos, 1901 and Zoogonoides Odhner, 1902 are closely related and represented by few species (Bray, Reference Bray, Bray, Gibson and Jones2008). To date, three cercariae belonging to these genera have been described: Zoogonus rubellus (Olson, 1869), Zoogonoides laevis Linton, 1940, and Zoogonoides viviparus (Olsson, 1868) (Stunkard, Reference Stunkard1938, Reference Stunkard1943; Køle, Reference Køle1976). The cercaria here described is provisionally assigned to Zoogonus, because it shares characteristics with Z. rubellus, such as a long prepharynx, short caeca and a small ventral sucker, when compared with species of Zoogonoides. Molecular results also show a high similarity between the sequences of the larva here described and Z. rubellus (AJ241804.1) (79% in ITS1 and 83% in ITS2). These results should be corroborated by sequencing the 18S and 28S regions, and by comparing with sequences available in GenBank, e.g. those from Z. viviparus, Deretrema nahaense Yamaguti, 1942, and Lepidophyllum steenstrupi Odhner, 1902.
Diversity assessment
A total of 12 larval digeneans were recorded in Puerto Deseado (southern site), 11 using gastropods as the first intermediate host and one, Gymnophalloides nacellae (Gymnophallidae), found only as metacercaria, the limpet acting only as second intermediate host, with a high prevalence (83.67%) (table 1). Of the remaining species, the most prevalent were the two microphallids (16.5% Microphallidae gen. et sp. 1 from Crepipatella dilatata and 9.2% Maritrema madrynense from Siphonaria lessonii), the renicolid (4.64% Renicolidae gen. et sp. 1 from Trophon geversianus) and the two lepocreadiids (3.49% Lepocreadiidae gen. et sp. 1 from C. dilatata and 3.76% Lepocreadiidae gen. et sp. 2 from Pareuthria fuscata).
A total of three larval digeneans were recorded in Puerto Madryn (northern site), all using snails as the first intermediate host (table 1), using a sampling scheme comparable to that used in the southern site (a total of six species were recorded previously by Gilardoni et al. (Reference Gilardoni2011)). As in the southern site, the most prevalent digeneans were the two microphallids (43.10% Microphallidae gen. et sp. 1 from C. dilatata and 26.25% M. madrynense from S. lessonii), but both prevalences were higher than at the southern site. Parorchis sp. from T. geversianus showed a low prevalence at both sites (0.66% and 0.59% in Puerto Deseado and Puerto Madryn, respectively).
The parasite species richness (taking into account all digenean species) was found to be higher in Puerto Deseado (12) than in the northern site, Puerto Madryn (6). Five digenean species occur at both localities (Hemiuroidea fam. gen. et sp., M. madrynense, Microphallidae gen. et sp. 1, Renicolidae gen. et sp. 1 and Parorchis sp.), seven occur only at Puerto Deseado (G. nacellae, Lepocreadiidae gen. et sp. 1, Lepocreadiidae gen. et sp. 2, Notocotylidae gen. et sp. 1, Renicolidae gen. et sp. 2, Schistosomatidae gen. et sp. and Zoogonus sp.), and only one is exclusive to Puerto Madryn (Diphterostomum sp.). Whether parasites occur at both sites or exclusively at one or other of the sites is determined by the exclusivity of snails that act as first intermediate hosts. In the same way, the parasite species richness (considering only the comparative sampling) and the gastropod species richness were higher in Puerto Deseado (11 and six, respectively) than Puerto Madryn (three and three, respectively).
The diversity index (H´) was higher in Puerto Deseado (t = −6.42; P < 0.05); this is explained mainly by the species richness, as the equitability was similar in both localities. Similar results were observed when parasite species that occurred at both sites were considered (t = −4.68; P < 0.05). The dominance index (D) was higher in Puerto Madryn because of the high prevalence of Microphallidae gen. et sp. from C. dilatata there. The Jaccard index value was low (table 1), indicating low similarity between the two parasite assemblages.
Discussion
This study describes five trematode species that parasitize six gastropod species in Puerto Deseado, on the Patagonian coast of Argentina: Lepocreadiidae gen. et sp. 1 from Crepipatella dilatata, Lepocreadiidae gen. et sp. 2 and Zoogonidae gen. et sp. 1 from Pareuthria fuscata, and Notocotylidae gen. et sp. 1 and Renicolidae gen. et sp. 2 from Nacella magellanica. The taxonomic determination of digenean larvae was supported by both molecular and morphological results. There are many morphological variations in cercariae that are constant within a species (or larger taxon); however, various genera within a family can exhibit the same type of cercaria (e.g. Maritrema madrynense and Microphallidae gen. et sp. 1, which are morphologically similar but genetically different). Molecular data provide a powerful tool with which to diagnose and study the life cycles of digeneans.
The species described in this study were recorded previously by Bagnato et al. (Reference Bagnato2015), summarizing a total of 12 digenean larval species found in Puerto Deseado. Of 11 digenean larvae that use snails as the first intermediate host, seven use shorebirds as the definitive host (two Microphallidae, one Notocotylidae, two Renicolidae, one Philophtalmidae and one Schistosomatidae) and the other four use fish (one Hemiuroidea, two Lepocreadiidae and one Zoogonidae) (Gibson et al., Reference Gibson, Jones and Bray2002; Jones et al., Reference Jones, Bray and Gibson2005; Bray et al., Reference Bray, Gibson and Jones2008). Other authors found shorebird digeneans to be the main parasitological component of marine coastal ecosystems (e.g. Lauckner, Reference Lauckner and Howell1987; Skirnisson & Galaktionov, Reference Skirnisson and Galaktionov2002) because most marine and coastal birds regularly visit the intertidal zone, where they rest or feed on coastal invertebrates and fish, which act as intermediate hosts for digeneans.
The two microphallid species were the most prevalent at both sites, and Microphallidae gen. et sp. 1 from C. dilatata was the dominant species in Puerto Madryn. Microphallid parthenites have been recorded as dominant species in other intertidal zones worldwide (e.g. Galaktionov & Bustnes, Reference Galaktionov and Bustnes1999; Granovitch et al., Reference Granovitch, Sergievsky and Sokolova2000; Fredensborg et al., Reference Fredensborg, Mouritsen and Poulin2006). Microphallids mainly use birds as definitive hosts, usually gulls (Larus spp.) on marine coasts (e.g. Yamaguti, Reference Yamaguti1975; La Sala et al., Reference La Sala2009; Diaz et al., Reference Diaz, Gilardoni and Cremonte2012). In this regard, Maritrema madrynense, one of the two microphallids recorded at both study sites, parasitizes the kelp gull Larus dominicanus as definitive host (Diaz & Cremonte, Reference Diaz and Cremonte2010; Bagnato et al., Reference Bagnato2015). Kelp gulls are characterized by varied and flexible foraging strategies (Yorio & Caille, Reference Yorio and Caille2004). The higher prevalence of microphallids could be explained by the higher abundance of kelp gulls at Puerto Madryn, which appears to be related to the high availability of food resources from an opencast garbage dump, a fishery dump and 18 fishery processing plants (Giaccardi & Yorio, Reference Giaccardi and Yorio2004).
The digenean assemblage harboured by gastropods from the southern study site (Puerto Deseado) had a higher species richness and diversity in comparison with the northern site (Puerto Madryn). Additionally, the parasite species described in this study were present only at the southern site, because of the geographical distribution of the mollusc hosts. The higher parasite diversity found at the southern site is partly explained by that site having higher mollusc diversity. Puerto Deseado is located in the Magellanic Biogeographical Province, which is characterized by a higher number of endemic taxa (Balech & Ehrlich, Reference Balech and Ehrlich2008) and a higher diversity of molluscs (Miloslavich et al., Reference Miloslavich and Riosmena-Rodríguez2016). Given the high specificity exhibited by trematodes in their first intermediate host, if a gastropod species is absent at the site then the digenean species is not present. However, even when we considered only the gastropod species that occur at both sites, parasite diversity was still higher at the southern site. A high diversity of molluscs and other invertebrates seems to attract shorebirds and fish (potential definitive hosts), which feed upon them in the intertidal, releasing the digenean eggs with their faeces. Other studies have reported that digenean diversity in molluscs that act as first intermediate hosts is directly related to the diversity of the definitive hosts (mainly birds in the intertidal zone) (e.g. Galaktionov & Bustnes, Reference Galaktionov and Bustnes1999; Huspeni et al., Reference Huspeni, Hechinger, Lafferty and Bortones2005; Hechinger & Lafferty, Reference Hechinger and Lafferty2005; Hechinger et al., Reference Hechinger, Lafferty and Kuris2008).
In the intertidal systems studied, only one of seven snail species surveyed (S. lessonii) was found to be the first intermediate host for three digenean species. Most of them hosted two species (C. dilatata, N. magellanica, P. fuscata and T. geversianus), and S. lateralis hosted only one. In other trematode–snail systems, in freshwater, marine or estuarine ecosystems, a gastropod species can host many digenean species. For example, Heleobia conexa (Cochliopidae) serves as first intermediate host in the cycles of at least 22 species of digeneans in the estuarine system of Mar Chiquita Albufera, Buenos Aires Province, Argentina (Merlo & Etchegoin, Reference Merlo and Etchegoin2011), and Littorina spp. (Littorinidae) are the most common snails in the intertidal zone of south-western Iceland, where they host 12 digenean species (Skirnisson & Galaktionov, Reference Skirnisson and Galaktionov2002). The low digenean species richness per gastropod species could be explained by the distribution and diversity of shorebirds (definitive hosts) feeding or resting in the intertidal zone (Smith, Reference Smith2001). Along the shoreline of the Patagonian coast of Argentina almost 45 species were recorded (Narosky & Yzurieta, Reference Narosky and Yzurieta1987; Yorio et al., Reference Yorio1998). However, as many as 182 bird species were recorded in Mar Chiquita Albufera (Ferrero, Reference Ferrero and Iribarne2001) and 70 in Iceland (Skirnisson & Galaktionov, Reference Skirnisson and Galaktionov2002).
The geographical distribution of larval digeneans in their molluscan hosts should depend on macro-scale factors (e.g. climate, host occurrence in the geographical regions studied) and local, micro-scale factors (e.g. distribution and abundance of intermediate and definitive hosts in the local habitat, and host abundance) (Skirnisson & Galaktionov, Reference Skirnisson and Galaktionov2002; Poulin & Mouritsen, Reference Poulin and Mouritsen2003). Our data suggest that both types of factors are acting in the rocky intertidal of Patagonia. A biogeographical pattern may result in higher mollusc diversity in the southern study site (which lies in the rich Magellanic Biogeographical Province), which results in higher digenean diversity, given their specificity for first intermediate hosts. However, the greater diversity of gastropods and other benthic species may further increase trematode diversity and abundance by attracting a greater abundance and diversity of shorebird and fish final hosts that disperse the digenean eggs and promote completion of their life cycles.
Acknowledgements
The authors thank Cristián Ituarte from MACN-CONICET for his help with sampling; Titina Zapata from Centro de Investigaciones, Universidad de la Patagonia Austral at Puerto Deseado, for kindly providing access to laboratory facilities; Jaime Groizard (Aluar S.A.) for the SEM photographs; and the anonymous reviewers of this article for their useful suggestions.
Financial support
This study was funded by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 1702, PICT 0841 and PICT 0653 Préstamo BID) and the Conservation, Food and Health Foundation.
Conflict of interest
None
Ethical standards
The molluscs were collected with permission from the Subsecretaría de Pesca, Santa Cruz province (Provincial Sample Permission Expte. 428.635/11).