Introduction
In the USA, breast cancer is the leading non-cutaneous cancer among women. The 5-year survival rate for breast cancer has improved from 74·6% during 1975–1979 to 90·8% in 2013–2019· 1,2 As outcomes for breast cancer patients have improved, reducing the late side treatment effects of treatment becomes paramount. One such toxicity is cardiac toxicity as major coronary events have been shown to increase by 7·4% for every additional 1 Gy of mean cardiac dose. Reference Darby, Ewertz and McGale3–Reference Darby, McGale and Taylor6 Despite the continuous improvements in radiotherapy methods, late cardiac toxicity remains a matter of concern, particularly for patients undergoing radiation treatment for left-sided breast cancer. Reference Chung, Oh and Chang7–Reference Yeboa and Evans9
Deep inspiration breath hold (DIBH) technique has emerged as a promising approach for reducing cardiac dose during radiotherapy by increasing the distance between the treatment target and the heart. Practically, this occurs as the patient maintains each deep inspiration, with multiple DIBHs during treatment. Numerous studies have explored the dosimetric benefits of using DIBH in left-sided breast cancer radiotherapy, comparing it with free breathing, and consistently reported reductions in mean doses to the lung, heart and left anterior descending artery (LAD). Reference Bruzzaniti, Abate and Pinnarò10–Reference Nissen and Appelt16 Application of DIBH in right-sided breast cancer treatment has also shown reductions in the maximum dose to the liver and the right coronary artery. Reference Pandeli, Smyth and David17
The reproducibility of the DIBH technique during treatment delivery is a crucial factor in determining if patients fully benefit from the dosimetric advantages seen in treatment planning studies. The ABC (Elekta AB, Stockholm, Sweden) technique is an approach to facilitate DIBH. This device utilises a spirometer to monitor and control the patient’s airflow, enabling the patient to maintain a predetermined inhaled volume and hold their breath consistently. The ABC device is widely used in various other treatment sites and plays a role in managing respiratory motion and reducing tumour motion, thus minimising treatment margins, and improving localisation accuracy, particularly in lung, liver and pancreatic stereotactic body radiation treatments. Reference Gough, Mowat and Sellman18–Reference Guo, Stephans and Woody20 In this study, we investigated the dosimetric impact of inter-fraction DIBH reproducibility assisted by the ABC device on inter-fractional cardiac dose. We calculated the variation in cardiac dose received during treatment compared to the planned dose as a surrogate measure to evaluate the reproducibility of the DIBH technique when assisted by the ABC device. While previous studies have assessed the reproducibility of ABC-assisted DIBH based on geometric or image-based metrics such as inhaled air volume, Reference Mohamed Yoosuf, Alhadab and Alshehri21 lung area variation, Reference Parsons, Joo and Iqbal22 portal image alignment Reference Mohamed Yoosuf, Alhadab and Alshehri21,Reference Kim, Little and Groen23 or field matching accuracy, Reference All, Zhao and Montalvo24 few have directly quantified the dosimetric variation to critical cardiac structures like the heart and LAD across treatment fractions. Our work aims to bridge that gap by focusing on actual dose deviation, which is ultimately the clinical target of DIBH. This approach provides a more direct assessment of how consistently ABC-DIBH delivers its intended cardiac sparing benefit throughout the treatment course.
Materials and Methods
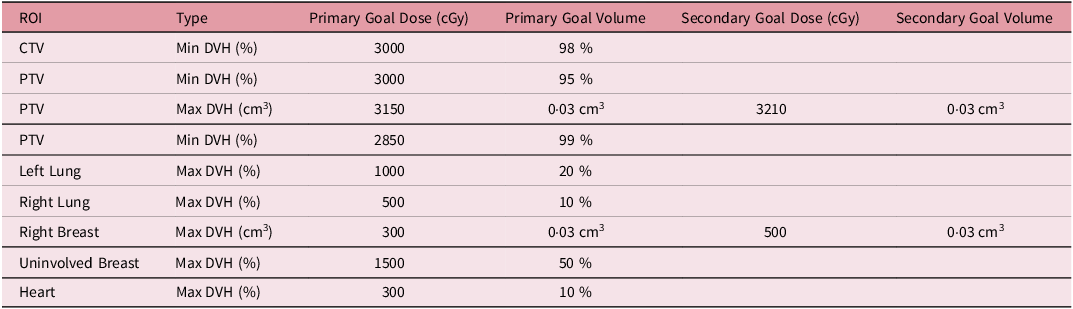
We retrospectively selected thirty-three patients randomly who had previously received PBI with 30 Gy in 5 fractions with DIBH at our institution between 2018 and 2020. The study was approved by the Institutional Review Board. Prior to simulation, these patients received training on how to perform deep inspiration breath hold utilising the ABC device. After training, the patient-specific inhalation volume threshold was determined and recorded. During the simulation, patients were positioned supine on an angled breast board, with their arms above their heads and their heads turned away from the ipsilateral breast side. A planning CT scan was acquired with a 3 mm slice thickness. The attending physician delineated the Clinical Target Volume (CTV) and Planning Target Volume (PTV), as well as nearby Organs at Risk (OARs), including the heart and lungs. LAD was contoured retrospectively for this study. Volumetric Modulated Arc Therapy (VMAT) plans were generated using 6 MV photon energy with Flattening Filter Free beams for 14 patients, and Flattened beams (FF) were used for 19 patients. The number of arcs (2–3) in each plan depended on the anatomy and complexity of the individual case. The plans were optimised to achieve at least 95% of the PTV receiving the prescription dose while adhering to the tolerance limits for critical structures. Planning objectives are summarised in Table 1.
Table 1. Planning objectives for left partial breast irradiation with prescription dose of 30 Gy in five fractions
During pre-treatment patient setup, kilo-voltage cone beam computed tomography (kV-CBCT) images were acquired with 3–4 breath-holds. The CBCT was aligned to the planning CT by matching soft tissue of the PTV. For this study, the heart and LAD contours on the CBCT were transferred from the planning CT after rigidly aligning the visible edge of the heart in both CBCT and planning CT. By applying the isocentre shifts from the treatment record, the heart and LAD contours from CBCTs were then transferred back to the planning CT to represent the treatment positions of these contours, thus allowing us to evaluate the treatment doses to the heart and LAD. Dose evaluation was performed by overlaying the planned 3D dose distribution onto the shifted contours based on their updated positions. During treatment, CBCT alignments were prioritised to the PTV coverage, aligning to the tumour cavity. For this study, all imaging registration, contour adjustments, and dose evaluations were performed using the MIM software (MIM Software Inc., Cleveland, OH, USA). 25 Figure 1 provides a schematic overview of the workflow, detailing the steps involved in determining contours of the heart and LAD on CBCTs and assessing the delivered doses on the planning CT. Figure 2 visually depicts the transfer of heart and LAD contours from the planning CT to the CBCT following cardiac alignment. Subsequently, these contours were shifted based on treatment imaging registration data (isocentre shifts) and then reintegrated into the planning CT for dose evaluation. Statistical analyses, including t-tests and Pearson correlation coefficients, were used to quantify the disparities between intended and actual administered mean and maximum doses to the heart and LAD.
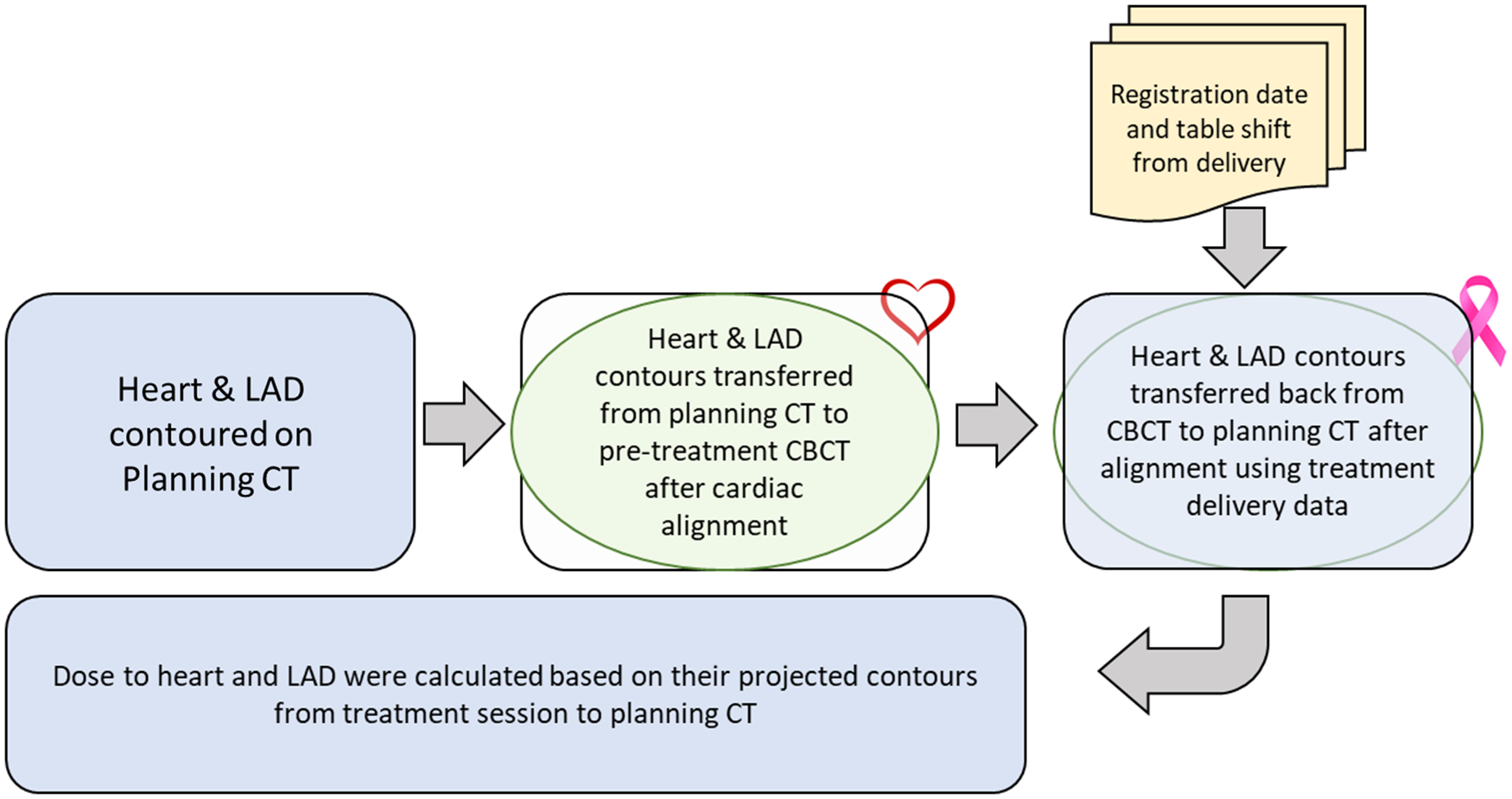
Figure 1. Workflow overview illustrating heart and LAD contour determination on CBCT scans and subsequent dose assessment on the planning CT.
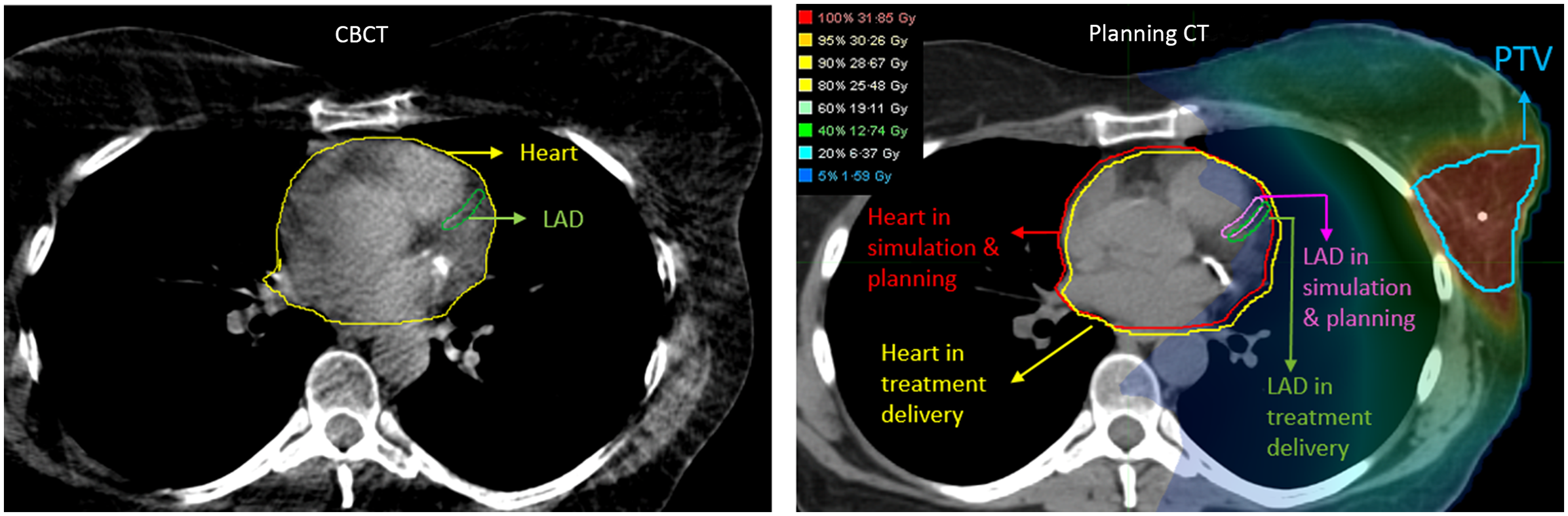
Figure 2. Left: Heart and LAD contours from the planning CT are rigidly registered and transferred to the CBCT based on cardiac alignment in MIM. Right: The heart and LAD contours are subsequently transferred back from the CBCT to the planning CT after rigid alignment, utilising treatment imaging registration data from delivery sessions to accurately represent their real positions during treatment.
Results
PTV volumes among the patients ranged from 42·3 cc to 824·5 cc, with an average value of 211·8 ± 144·9 cc. The planned mean heart dose for the thirty-three patients varied from 14·0 cGy to 201·0 cGy, with an average value of 67·7 ± 40·3 cGy. Overall, the mean cardiac dose increased by 2·4 ± 6·6 cGy when averaged over all patients through their entire course of treatments. Importantly, no patient exceeded a mean heart dose deviation of 5·8 cGy beyond the planned value in any individual treatment fraction. The highest mean heart dose deviation among patients throughout the entire treatment course was 16·4 cGy. The planned maximum dose to the heart ranged from 79·0 cGy to 1450·0 cGy, with an average of 449·8 ± 321·6 cGy. Across all patients throughout their entire treatment course, the average increase in maximum cardiac dose was 23·3 ± 58·0 cGy. The most substantial increase occurred in the maximum heart dose, exceeding the planned dose by 82·4 cGy in a single fraction, resulting in an overall increase of 264·8 cGy throughout the entire course of treatment when averaged over all five fractions of the patient treatment course.
The planned mean LAD dose exhibited considerable variability among the thirty-three patients, spanning from 21·0 cGy to 759·0 cGy, with an average value of 158·4 cGy ± 171·2 cGy. Throughout the entire treatment course for all patients, the mean LAD dose increased on average by 4·8 ± 18·5 cGy. Throughout the treatment, no patient received a mean LAD dose exceeding 56·2 cGy beyond the planned value in any single fraction, and a cumulative increase of 54·0 cGy over the entire course of treatment. The planned maximum LAD doses ranged from 41·0 cGy to 1733·0 cGy, with an average value of 331·8 cGy ± 361·9 cGy. Throughout all patients’ treatment courses, the average rise in maximum LAD dose was 17·0 ± 51·0 cGy. When compared to the delivered doses, the most substantial increase in the maximum LAD dose occurred, reaching 181·8 cGy in any single fraction. On average across all five treatment fractions, the greatest deviation in LAD maximum dose among patients resulted in an increase of 160·2 cGy.
No significant difference between the planned and delivered mean and maximum doses to the heart and LAD was noted. Additionally, a strong positive correlation was observed between the planned and delivered doses to the heart and LAD, with a Pearson correlation coefficient of 0·99 for both mean and maximum doses (p < 0·05). Figure 3 illustrates the mean and maximum doses delivered to the heart and LAD in each fraction compared to the expected dose in the treatment plan. The red dashed best-fitted line closely aligns with the bisector line, demonstrating a strong agreement between the planned and delivered doses. Figure 4 displays the differences between the mean and maximum doses delivered to the heart and LAD throughout the entire course of treatment and the expected doses in the treatment plan for all patients.
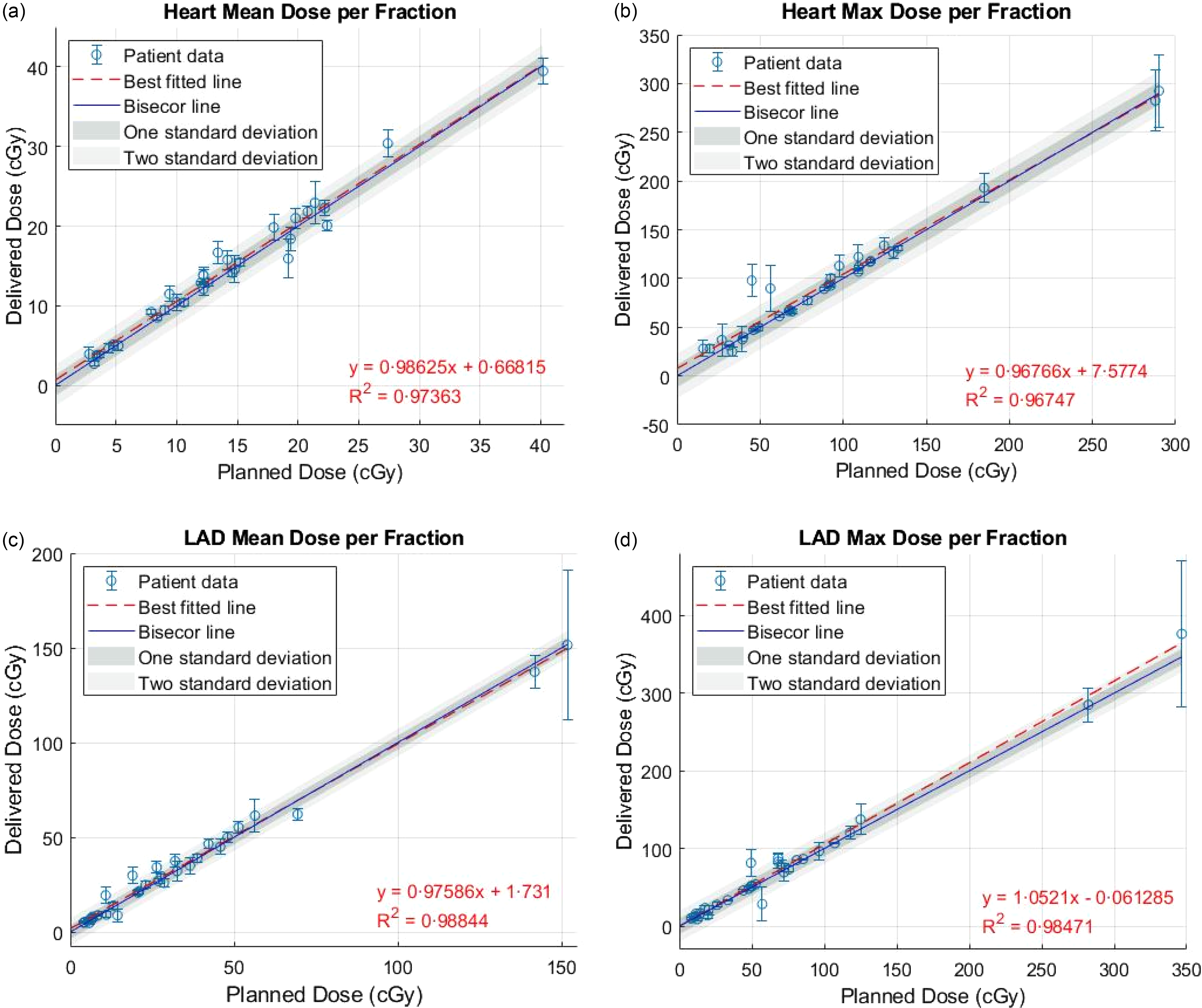
Figure 3. Comparison of mean and maximum doses delivered to the heart and LAD in each treatment fraction with the expected doses from the treatment plan.
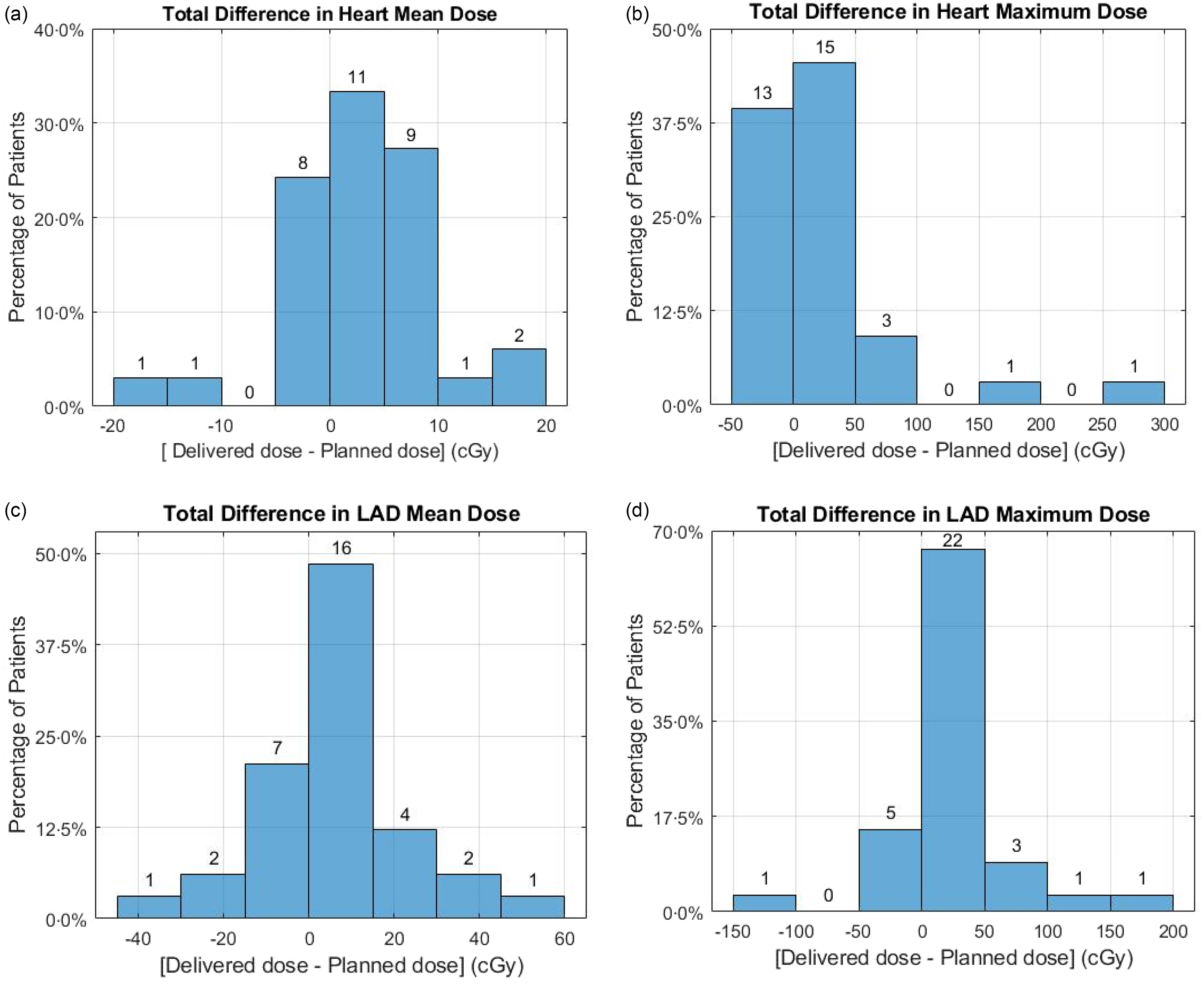
Figure 4. Comparison of delivered versus expected doses for heart and LAD throughout the entire treatment course.
Discussion
In this study, we examined the dosimetric impact of inter-fractional reproducibility in DIBH assisted by the ABC device on cardiac radiation dose for PBI. Analysis of cardiac and LAD dose variations across treatment sessions provided insight into the reproducibility of DIBH with ABC assistance in achieving planned dose levels. The delivered mean heart and LAD doses showed minimal deviations from the intended values. The highest mean heart dose deviation was 16·4 cGy, while the greatest increase in mean LAD dose among patients was 54·0 cGy on average across all treatment fractions. The maximum doses displayed more substantial deviations, with a 264·8 cGy increase in maximum heart dose and a 160·2 cGy increase in maximum LAD dose recorded as the greatest deviations observed among patients when averaged over the entire course of treatment. A strong positive correlation (Pearson correlation coefficient of 0·99, p < 0·05) between planned and delivered doses underscores the reliability and reproducibility of DIBH with ABC assistance.
The utilisation of DIBH with the ABC device plays a crucial role in minimising cardiac exposure during left-sided breast cancer radiotherapy. While several online breath-hold verification techniques have emerged as promising tools for treating various sites, Reference Vasina, Kong and Greer26–Reference Chen and Wang31 the use of the ABC device as the primary tool for providing reproducible breath-holds is still widely employed in the treatment of breast cancer patients, particularly for left-sided breast treatment, and many patients still benefit from this technique. The primary objective of implementing DIBH in left breast cancer treatment is to spare the heart from excessive radiation exposure. Our study reaffirms the efficacy of this technique in achieving precise dose delivery while mitigating risks to critical cardiac structures. Additionally, the ABC device has demonstrated high consistency in controlling lung volumes, highlighting its reliability in delivering reproducible breath-holds. Reference Kaza, Dunlop and Panek32 This precision enhances the safety and accuracy of radiation delivery, reinforcing the effectiveness of the ABC device in protecting critical OARs. In contrast, DIBH patient setup based solely on surface guided radiation therapy (SGRT), without complementary imaging guidance, is inherently dependent on patient-specific factors such as setup error, surface irregularities, respiratory behaviour and potential breast deformation. Reference Bright, Foster and Hampton33–Reference Li, Lu and O’Grady35 In such workflows, the absence of imaging-based verification can compromise PTV coverage and adversely affect OAR doses, potentially exceeding accepted planning objectives. Reference Rudat, Shi and Zhao36,Reference Mankinen, Virén and Seppälä37
Another important takeaway comes from the method. The technique used to assess inter-fractional changes to dose for organs at risk holds potential as an in-house online verification method for verifying inter-fractional motion in the treatment of other sites, such as lung, liver and pancreas, in addition to left breast cancer. It can be effectively utilised in treatments that employ kV-CBCT for patient setup. By establishing a dosimetric variation threshold for nearby structures, our method can serve as a valuable secondary check to ensure the quality of patient breath-hold, complementing the threshold set on the ABC device. This approach enables the accurate monitoring of inter-fractional motion, providing enhanced confidence in treatment delivery.
While the ABC device offers consistent and reproducible breath-hold control, patient comfort and usability can vary. Some studies have reported that a subset of patients may find the ABC apparatus restrictive or uncomfortable, particularly those with anxiety or respiratory limitations. Reference Cashell, Qadeer and Rosewall38 In such cases, voluntary DIBH techniques guided by SGRT may offer a more tolerable alternative. Reference Ranger, Dunlop and Grimwood39 These considerations underscore the importance of tailoring the breath-hold method to individual patient needs and highlight the role of shared decision-making in clinical practice.
There are limitations to our study. A key limitation was the necessity to transfer the contours from the Planning CT to the CBCT after cardiac alignment, instead of directly contouring the heart and LAD on the CBCT. This was primarily due to the inadequate imaging quality of the CBCT, making contouring of the LAD unfeasible. To ensure consistency and report the mean dose metric accurately, we utilised the same structure contours from the Planning CT. Additionally, due to the limited field of view in the CBCT and the positioning of the PTV, some slices of the heart contour in the superior or inferior regions were not captured by the CBCT and could not be delineated. As a result, transferring the contours from the CT to the CBCT was the only viable option to delineate the entire heart. A further limitation in this study was that the dosimetric values reported were derived from overlaying the planned 3D dose distribution onto the shifted contours without recalculating the dose, which could have yielded a more accurate dose calculation, particularly for maximum dose estimates, where summing the maximum doses from each fraction likely overestimates both the maximum dose and its variability.
Another important consideration is that the delivered dose values reported in this study are derived from mapping the planned dose distribution onto the patient’s anatomy at the time of CBCT-based image verification. While this method provides a practical estimate of the dose under daily anatomical variations, it does not represent the actual dose accumulated during the entire treatment delivery. Intra-fractional motion and physiological changes occurring after imaging are not captured in this analysis. As such, the term delivered dose in this context reflects the estimated dose at the moment of image guidance rather than the true delivered dose. Accounting for such intra-fractional variability would require real-time or post-treatment dose reconstruction, which is beyond the scope of this study.
Despite these limitations, our approach allowed us to evaluate the cardiac dose with reasonable accuracy.
Conclusions
Based on the data obtained in this study, it is evident that the utilisation of the ABC device aids in achieving effective reproducibility of DIBH during partial left breast radiotherapy. This reproducibility, in turn, contributes to successful cardiac sparing. The findings provide robust evidence supporting the use of DIBH assisted by the ABC device as a valuable technique for minimising radiation exposure to the heart and LAD in left breast cancer treatment.
Author contributions
Mojtaba Moazzezi: Conceptualisation, Methodology, Data Analysis, Writing - Original Draft. Ananta Raj Chalise: Data Collection, Data Analysis. Zhexuan Zhang: Data Collection. Ahmed Mohamed Kamal Ibrahim Halima: Cardiac Structure Delineation. Bingqi Guo, PhD: Supervision. Rahul Tendulkar, MD: Supervision. Chirag Shah, MD: Reviewing, Editing. Ping Xia, PhD: Project Development and Definition, Reviewing, Editing.
Financial support
None.
Competing interests
Chirag Shah serves as a scientific consultant for ImpediMed, PreludeDX, and Videra Surgical. He has received grants from Varian Medical Systems, and PreludeDX.







