Published online by Cambridge University Press: 29 May 2018
The new mineral ammoniomathesiusite (NH4)5(UO2)4(SO4)4(VO5)·4H2O, was found in the Burro mine, San Miguel County, Utah, USA, where it occurs as a secondary phase on asphaltum/quartz matrix in association with ammoniozippeite, gypsum, jarosite and natrozippeite. The mineral forms pale yellow to greenish-yellow prisms, up to ~0.3 mm long, with pale-yellow streak and bright yellow–green fluorescence. Crystals are transparent and have vitreous lustre. The mineral is brittle, with Mohs hardness of 2½, stepped fracture and two cleavages: excellent on {110} and good on {001}. The calculated density is 3.672 g/cm3. Ammoniomathesiusite is optically uniaxial (–) with ω = 1.653(2) and ε = 1.609(2) (white light). Pleochroism is: O = green-yellow, E = colourless; O > E. Electron microprobe analyses yielded the empirical formula [(NH4)4.75(UO2)4(SO4)4(VO5)·4(H2.07O). The five strongest powder X-ray diffraction lines are [dobs Å(I)(hkl)]: 10.57(46)(110), 7.10(62)(001), 6.41(100)(101), 3.340(35)(240) and 3.226(44)(141). Ammoniomathesiusite is tetragonal, P4/n with a = 14.9405(9), c = 7.1020(5) Å, V = 1585.3(2) Å3 and Z = 2. The structure of ammoniomathesiusite (R1 = 0.0218 for 3427 I > 2σI) contains heteropolyhedral sheets based on [(UO2)4(SO4)4(VO5)]5– clusters. The structure is identical to that of mathesiusite, with 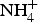 ${\rm NH}_{\rm 4}^{\rm +} $ in place of K+.
${\rm NH}_{\rm 4}^{\rm +} $ in place of K+.
Associate Editor: Juraj Majzlan