Published online by Cambridge University Press: 05 July 2018
Hylbrownite, ideally Na3MgP3O10·12H2O, the second known triphosphate mineral, is a new mineral species from the Dome Rock mine, Boolcoomatta Reserve, Olary Province, South Australia, Australia. The mineral forms aggregates and sprays of crystals up to 0.5 mm across with individual crystals up to 0.12 mm in length and 0.02 mm in width. Crystals are thin prismatic to acicular in habit and are elongate along [001]. Forms observed are {010}, {100}, {001}, {210} and {201}. Crystals are colourless to white, possess a white streak, are transparent, brittle, have a vitreous lustre and are nonfluorescent. The measured density is 1.81(4) g cm−3; Mohs' hardness was not determined. Cleavage is good parallel to {001} and to {100} and the fracture is uneven. Hylbrownite crystals are nonpleochroic, biaxial (−), with α = 1.390(4), β = 1.421(4), γ = 1.446(4) and 2Vcalc. = 82.2°. Hylbrownite is monoclinic, space group P21/n, with a = 14.722(3), b = 9.240(2), c = 15.052(3) Å, β = 90.01(3)°, V = 2047.5(7) Å3, (single-crystal data) and Z = 4. The strongest lines in the powder X-ray diffraction pattern are [d (Å)(I)(hkl)]: 10.530(60)(10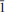 ,101), 7.357(80)(200), 6.951(100)(11
,101), 7.357(80)(200), 6.951(100)(11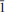 , 111), 4.754(35)(10
, 111), 4.754(35)(10 , 103), 3.934(40)(022), 3.510(45)(30
, 103), 3.934(40)(022), 3.510(45)(30 , 303), 3.336(35)(41
, 303), 3.336(35)(41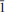 , 411). Chemical analysis by electron microprobe gave Na2O 16.08, MgO 7.08, CaO 0.43, P2O5 37.60, H2Ocalc 38.45, total 99.64 wt.%. The empirical formula, calculated on the basis of 22 oxygen atoms is Na2.93Mg0.99Ca0.04P2.99O9.97·12.03H2O. The crystal structure was solved from single-crystal X-ray diffraction data using synchrotron radiation (T = 123 K) and refined to R1 = 4.50% on the basis of 2417 observed reflections with F0 > 4 σ(F0). [Mg(H2O)3P3O10] clusters link in the b direction to Naφ6 octahedra, by face and corner sharing. Edge sharing Naφ6 Octahedra and Naφ7 polyhedra form Na2O9 groups which link via corners to form chains along the b direction. Chains link to [Mg(H2O)3P3O10] clusters via corner-sharing in the c direction and form a thick sheet parallel to (100). Sheets are linked in the a direction via hydrogen bonds.
, 411). Chemical analysis by electron microprobe gave Na2O 16.08, MgO 7.08, CaO 0.43, P2O5 37.60, H2Ocalc 38.45, total 99.64 wt.%. The empirical formula, calculated on the basis of 22 oxygen atoms is Na2.93Mg0.99Ca0.04P2.99O9.97·12.03H2O. The crystal structure was solved from single-crystal X-ray diffraction data using synchrotron radiation (T = 123 K) and refined to R1 = 4.50% on the basis of 2417 observed reflections with F0 > 4 σ(F0). [Mg(H2O)3P3O10] clusters link in the b direction to Naφ6 octahedra, by face and corner sharing. Edge sharing Naφ6 Octahedra and Naφ7 polyhedra form Na2O9 groups which link via corners to form chains along the b direction. Chains link to [Mg(H2O)3P3O10] clusters via corner-sharing in the c direction and form a thick sheet parallel to (100). Sheets are linked in the a direction via hydrogen bonds.