Published online by Cambridge University Press: 05 July 2018
The ideal end-members reddingite, Mn3 2+ H2 O)3(PO4)2, phosphoferrite, 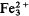 (H 2O)3(PO4)2, and kryzhanovskite,
(H 2O)3(PO4)2, and kryzhanovskite, 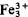 (OH)3(PO4)2 form a complex triple series. Similarities in crystal axes and pronounced differences in site preferences have led to erroneous indexing of the powder data and subsequent errors in cell refinements. Writing the general formula M(1)M(2)2[(H2O),(OH)]3(PO4)2, the following end-member names apply:
(OH)3(PO4)2 form a complex triple series. Similarities in crystal axes and pronounced differences in site preferences have led to erroneous indexing of the powder data and subsequent errors in cell refinements. Writing the general formula M(1)M(2)2[(H2O),(OH)]3(PO4)2, the following end-member names apply:

Type landesite,Ca0.4Mg1.2 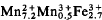 (OH)3.2(H2O)8.8(PO4)8.0, has a = 9.458(3) Å, b = 10.185(2) Å, c = 8.543(2) Å, space group Pbna. R = 5.2% for 1821 independent reflexions (Mo-Kα radiation). The distance averages are M(1)-O = 2.098 Å, M(2)-O = 2.205 Å, P-O = 1.539 Å. Cotype kryzhanovskite,Ca0.5Mg0.4
(OH)3.2(H2O)8.8(PO4)8.0, has a = 9.458(3) Å, b = 10.185(2) Å, c = 8.543(2) Å, space group Pbna. R = 5.2% for 1821 independent reflexions (Mo-Kα radiation). The distance averages are M(1)-O = 2.098 Å, M(2)-O = 2.205 Å, P-O = 1.539 Å. Cotype kryzhanovskite,Ca0.5Mg0.4 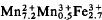 (OH)7.3(H4O)4.7(PO4)8.0, has a = 9.450(2) Å, b = 10.013(2), c = 8.179(2). R = 7.2% for 1,703 independent reflexions (Mo-Kα radiation). The distance averages are M(1)-O = 2.017 Å, M(2)-O = 2.115, P-O = 1.542.
(OH)7.3(H4O)4.7(PO4)8.0, has a = 9.450(2) Å, b = 10.013(2), c = 8.179(2). R = 7.2% for 1,703 independent reflexions (Mo-Kα radiation). The distance averages are M(1)-O = 2.017 Å, M(2)-O = 2.115, P-O = 1.542.
Computed powder pattern intensities from the structure data admitted extensive revisions of earlier published Miller indices and revised powder data are presented.