Published online by Cambridge University Press: 24 July 2023
Rewitzerite, K(H2O)Mn2(Al2Ti)(PO4)4[O(OH)](H2O)10⋅4H2O, is a new monoclinic member of the paulkerrite group, from the Hagendorf-Süd pegmatite, Oberpfalz, Bavaria, Germany. It was found in specimens of altered zwieselite, in association with rockbridgeite. Rewitzerite forms clusters of colourless elongated hexagonal-shaped prisms, up to 0.1 mm long. The crystals are flattened on {010} and elongated along [100], with forms {010}, {001}, {111} and {$\bar{1}$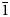 11}. The calculated density is 2.33 g⋅cm–3. Optically, rewitzerite crystals are biaxial (+), with α = 1.585(2), β = 1.586(2), γ = 1.615(2) (measured in white light) and 2V(meas) = 25(2)°. The empirical formula from electron microprobe analyses and structure refinement is A1[K0.77(H2O)0.23]A2[H2O] M1(Mn2+0.82Mg0.64Fe3+0.43□0.11)Σ2.00 M2+M3(Al1.51Ti4+1.06Fe3+0.43)Σ3.00(PO4)4 X[(OH)0.54F0.42O1.04]Σ2.00(H2O)10⋅4H2O, where □ = vacancy.
11}. The calculated density is 2.33 g⋅cm–3. Optically, rewitzerite crystals are biaxial (+), with α = 1.585(2), β = 1.586(2), γ = 1.615(2) (measured in white light) and 2V(meas) = 25(2)°. The empirical formula from electron microprobe analyses and structure refinement is A1[K0.77(H2O)0.23]A2[H2O] M1(Mn2+0.82Mg0.64Fe3+0.43□0.11)Σ2.00 M2+M3(Al1.51Ti4+1.06Fe3+0.43)Σ3.00(PO4)4 X[(OH)0.54F0.42O1.04]Σ2.00(H2O)10⋅4H2O, where □ = vacancy.
Rewitzerite has monoclinic symmetry with space group P21/c and unit-cell parameters a = 10.444(2) Å, b = 20.445(2) Å, c = 12.2690(10)Å, β = 90.17(3)°, V = 2619.8(6) Å3 and Z = 4. The crystal structure was refined using synchrotron single-crystal data to wRobs = 0.068 for 5894 reflections with I > 3σ(I). The crystal structure has the same topology as that for orthorhombic paulkerrite-group minerals but differs primarily in having an ordering of K+ and H2O molecules in different A sites, whereas they are disordered at a single A site in the orthorhombic members of the group.
Associate Editor: Michele Dondi