Introduction
Over the past decade, three-dimensional (3D) printing and related additive manufacturing (AM) technologies have started to rapidly displace traditional manufacturing in a wide range of industries and applications, from automotive to aerospace to medical devices. Reference Huang, Liu, Mokasdar and Hou1–Reference Bandyopadhyay, Bose and Das5 The capability to accurately deposit materials layer-by-layer in 3D space with precision of <100 μm has proven to be truly disruptive. The advantages of 3D printing include the ability to (1) rapidly iterate designs at low cost, (2) easily customize designs for different applications, and (3) generate complex designs that are difficult or impossible to achieve with machining, injection molding, or other traditional manufacturing approaches.
While there have been advances in hardware systems, many of the major improvements in the quality of 3D printed parts are due to advances in the materials used. Whether metals, ceramics, or polymers, it is critical that the materials have consistent chemical and physical properties in order to enable reliable fabrication of parts with high fidelity. This, in combination with process optimization, provides control over microstructure of the material and thus mechanical properties of the 3D printed part. While many established materials have been adapted and reformulated to work in AM processes, this often requires compromises in materials properties. Instead, it is the development of new materials specifically designed to take advantage of AM processes that represent the most exciting research in this area.
In 2015, the MRS Bulletin issue on “3D printing of biomaterials” provided a broad overview of the field, with a major focus on the opportunities and challenges associated with 3D printing of established, US Food and Drug Administration (FDA)-approved biomaterials for fabricating medical devices. Reference Bandyopadhyay, Bose and Das5 A primary advantage for these applications is the ability to easily customize the geometry to match patients’ unique anatomical structure, based on computed tomography (CT) or magnetic resonance imaging (MRI) imaging data. This 3D information, in combination with new software tools for modeling and simulation, enables the design of high-performance and patient-specific medical devices.
Over the past few years, multiple companies have brought products to market, including examples such as 3D printed skull plates composed of polyetherketoneketone or titanium, 6 surgical guides and implants for reconstructive surgery, Reference Shafiee and Atala7 and orthopedic implants for vertebral fixation and other applications. Reference Letourneau, Davies, Tabibkhoei, Daubert, Beck, Schryber, Madagan, Baird, Jacobson, Maiden and Quinn8 In general, these devices use FDA-approved materials for the same application, just using a different manufacturing technique. Thus, they can achieve US FDA clearance using a 510(k) approval process, 9,Reference Sastry10 where companies demonstrate substantial equivalence in device performance and that the AM approach does not alter safety or efficacy. There are similar medical-device approval pathways in other countries, such as the CE mark in the EU.
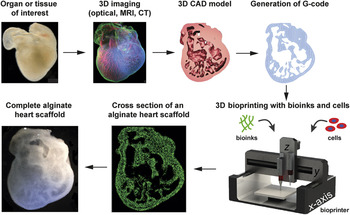
The next major anticipated advance is 3D bioprinting of materials or cells for engineering tissues and biological systems. The primary advantage of AM for this application is control of scaffold architecture and material composition in 3D space. The use of cells and a broad range of biomaterials enables the fabrication of more complex tissues and biohybrid systems that can combine biologic and synthetic components. The basic 3D bioprinting paradigm ( Figure 1 ) Reference Hinton, Jallerat, Palchesko, Park, Grodzicki, Shue, Ramadan, Hudson and Feinberg11 seeks to recreate tissue or organ structure and function by imaging the native architecture in 3D and then converting this into a 3D computer-aided design (CAD) model that can then be printed with the appropriate cells or bioinks. Reference Hinton, Lee and Feinberg12 Specifically, bioinks are the materials that are deposited in 3D space by the printer and are an area of active research and development, as will be explained later in this article.

Figure 1. An overview of the general 3D bioprinting process with the goal of engineering a specific tissue or organ structure. The tissue or organ of interest is identified and can be explanted or characterized in vivo using optical, magnetic resonance imaging (MRI), or computed tomography (CT) 3D imaging techniques. The 3D imaging data are then segmented into a computer-aided design (CAD) file, with the type of material and cellular information dictated by the resolution and specificity of the imaging technique. The CAD model is then converted to a stereolithography (STL) file, and a slicer program is used to convert the STL into G-code, which is a specific instruction set that defines how the printer moves in 3D space to build the structure. The G-code is then sent to the 3D bioprinter, where bioinks and/or cells are used to print the structure. Finally, examples are shown of a 3D heart scaffold bioprinted from alginate based on 3D confocal imaging of an embryonic stage chick heart. Adapted with permission from Reference Reference Hinton, Jallerat, Palchesko, Park, Grodzicki, Shue, Ramadan, Hudson and Feinberg11. © 2015 AAAS.
A range of imaging modalities can be used to create 3D data sets, including optical techniques such as confocal microscopy and optical coherence tomography, as well as medical imaging methods such as MRI and CT. The latter enables in vivo imaging in live patients, providing the potential to engineer truly patient-specific tissue for disease or trauma repair. Conversion of medical-imaging data to 3D CAD models is now standard clinical practice for surgical planning, and similar techniques can be applied for bioprinting. However, the need to maintain cell viability and preserve the activity of biological molecules places severe restrictions on the range of environmental and processing conditions that can be used during fabrication. This is one of the primary reasons that new biomaterials and improved printing approaches are needed. For example, cells must be maintained between 4–37°C with minimal shear stress and sufficient oxygen during most biofabrication strategies. Globular biological proteins can easily denature outside of their native operating ranges. This issue of MRS Bulletin highlights current 3D bioprinting approaches and both the advances and challenges that remain in bioinks, support materials, and multimaterials printing, as well as applications in tissue engineering and organs-on-a-chip.
Three-dimensional bioprinting approaches
Three-dimensional bioprinting is uniquely positioned to engineer complex, biological systems and to recreate the multiscale structure–function hierarchy found in many tissues and organs. Unlike subtractive manufacturing, which has dominated object fabrication for decades, 3D bioprinting provides the researcher access to every (x, y, z) Cartesian coordinate within a given volume. For example, microfabrication techniques based on photolithography, such as soft lithography and microfluidics, have been widely used to engineer biocompatible systems with spatial resolution from ∼1 μm up to 1 mm. However, critical tradeoffs must be considered when designing a bioprinting system or workflow. There are many fabrication technologies that excel at specific length scales, such as electrospinning to generate nanofibers and microfabrication, but very few that can span the full range of microscale to macroscale. This requires specific cell types to be positioned and interconnected precisely in 3D space, mimicking native tissue architecture and physiology. Yet, an increase in feature resolution is always accompanied by dramatic increases in cost due to the precision hardware and software required to achieve such scales, and the time to fabricate a given volume of material thereby increases.
Researchers must first consider the end goal for the desired tissue construct, then work backward in a retrosynthetic analysis to determine the specific materials to use, the fabrication technology, and the desired resolution, in order to fabricate and validate the tissue prototype. For example, in heart muscle the macroscale beating (contraction) is produced by the coordinated actuation of trillions of motor proteins at the molecular scale. Reference Bers13 Since no readily available technology can deposit single molecules or structured tissues at the scale of native organs, researchers must choose the scale at which they would like to work.
There are four major types of 3D printing techniques commonly adapted for biofabrication ( Figure 2 ). Reference Miller and Burdick14,Reference Miller15 In extrusion 3D printing, named fused deposition modeling (FDM) and trademarked by the inventor Stratasys in the 1980s, a computer controls the location of a syringe and its extrudate to deposit liquid-like materials that solidify in situ and thereby enable the buildup of a 3D structure. Cells can be mixed with aqueous extrudate, commonly referred to as “bioink,” to make structures from this thread-like deposition. In inkjet printing, consumer two-dimensional inkjet cartridges are adapted for bioprinting by exchanging ink dyes in the cartridge for biomaterials and living cells. Significant shear stresses are exerted on the fluid droplets, but biocompatible ejection rates have been identified. In selective laser sintering (SLS) a bed of powder is sintered or melted by a translating laser, and then a new layer of powder is swept atop the nascent part to enable buildup of a structured part. Due to the heat involved and the dry powders used, this technique is not directly compatible with deposition of cells until after the final part has been fabricated and submerged into aqueous solutions and seeded with cells. Finally, a stereolithography apparatus (SLA) utilizes photocurable resins and patterned light to selectively solidify material in a layer-by-layer fashion. Recently, researchers have begun adapting photocurable hydrogels and biocompatible photopolymerization to build soft gels that entrap living cells during fabrication.

Figure 2. Biomanufacturing is typically approached using one of these methods previously developed for plastic 3D printing. Extrusion printing and inkjet printing utilize liquid-like precursors that can solidify in situ after extrusion or ejection and can also encapsulate cells. Selective laser sintering binds or melts dry powders one layer at a time. Finally, stereolithography uses photosensitive aqueous solutions that can be polymerized with patterned light and can also encapsulate cells. Reference Miller and Burdick14 Courtesy of J. Albritton and J. Miller.
In this issue: Materials-focused challenges and applications in bioprinting
The articles in this issue of MRS Bulletin discuss current advances in the field of 3D bioprinting, the materials-focused challenges that must be overcome to achieve new capabilities, and recent applications in the areas of tissue engineering and organs-on-chip. In their article in this issue, Rutz et al. Reference Rutz, Lewis and Shah16 discuss the development of next-generation bioinks and how the material properties can be tuned to optimize cell viability during and after the printing process. They highlight how bioink properties are altered by the presence and concentration of cells, and that this must be properly accounted for in order to achieve good print fidelity and cell viability.
In their article, O’Bryan et al. Reference O’Bryan, Bhattacharjee, Niemi, Blalchandar, Baldwin, Ellison, Taylor, Sawyer and Angelini17 focus on recent advances in the 3D bioprinting of soft cells and materials, broadly referred to as soft matter, using sacrificial materials. The authors discuss the material properties required for the sacrificial bioinks that serve as the temporary support structure that can be dissolved later to produce vascular channels, as well as for gel-like sacrificial support materials within which soft matter can be printed (embedded) and then later released.
In their article, De Maria et al. Reference De Maria, Vozzi and Moroni18 consider how to implement more advanced bioprinting systems that use multiple extruders to fabricate multimaterial, heterogeneous, and multicellular 3D structures. This is a critical capability as researchers seek to use 3D bioprinting to recreate the complexity of native tissues and organs.
Moving toward application, the article by Kilian et al. Reference Kilian, Ahfeld, Akkineni, Lode and Gelinsky19 in this issue highlights the current progress in and outstanding challenges for 3D bioprinting of volumetric tissues and organs. This requires consideration of the individual materials properties of the bioinks, support materials, and cells, as well as their interactions during and after printing.
Finally, the article by Huang et al. Reference Huang, Zhang and Liu20 in this issue discusses the application of bioprinting toward engineering 3D culture models and organ-on-a-chip microfluidic systems. The authors highlight the capabilities and limitations of bioprinting compared to established microfabrication approaches, and how advances in multimaterial deposition and higher resolutions are critical for printing microphysiological tissue models.
This issue offers a materials-focused overview of the current 3D bioprinting field and highlights how understanding the properties of cells, bioinks, and sacrificial materials, and the dynamic interactions between these components is critical for future applications in volumetric, engineered tissues, and organ-on-a-chip systems.
Emerging and future trends
Open-source 3D printing platforms for low-cost biomaterials development and biofabrication
The growth in 3D bioprinting research has been driven in part by the increased accessibility of the underlying AM technology platforms. A decade ago, most research groups either adapted existing industrial-grade rapid prototyping systems for bioprinting applications, or built their own custom-designed 3D bioprinters from scratch. In both cases, the specialized expertise in terms of hardware and software, in combination with the high cost of these systems, was a significant barrier to entry. However, this earlier work resulted in a number of clear successes that demonstrated the potential of 3D bioprinting as a biofabrication platform. Reference Michna, Wu and Lewis21–Reference Skardal, Mack, Kapetanovic, Atala, Jackson, Yoo and Soker26
This spurred the development of commercial 3D bioprinters to address the needs of the growing research community, notable examples being the Organovo NovoGen MMX Bioprinter and the Envisiontec 3D-Bioplotter ( Figure 3a). This has increased accessibility by providing turnkey bioprinting platforms, but these systems are still cost prohibitive, typically >USD$100,000. A number of research groups and core facilities at major research universities have purchased these commercial systems, but the install base is relatively small.

Figure 3. Examples of commercial and open-source-based 3D bioprinters. (a) The Envisiontec Bioplotter is a commercial 3D bioprinter. (b) A low-cost, consumer-grade MakerBot Replicator 3D printer converted into a 3D bioprinter by replacing the thermoplastic extruder with (c) a custom-designed open-source dual-syringe pump extruder. Reference Hinton, Jallerat, Palchesko, Park, Grodzicki, Shue, Ramadan, Hudson and Feinberg11 (d) A standard laser cutter converted into an open-source selective laser sintering printer using a custom-designed powder bed (e) capable of printing using poly(caprolactone) and nylon. Reference Kinstlinger, Bastian, Paulsen, Hwang, Ta, Yalacki, Schmidt and Miller33 (f) The BioBots 2 is a lower cost commercial 3D bioprinter based in part on the RepRap open-source 3D printer platform.
In the last five years, the emergence of the open-source and “maker” communities has led to the growth of low-cost 3D bioprinting platforms with widespread accessibility. This transition has been driven by a number of fundamental 3D printing patents expiring, enabling established AM technologies to be integrated into a new generation of low-cost systems. The start of this process was the expiration of the patent for FDM in 1999, which described the process of layer-by-layer 3D printing using a thermoplastic filament. Reference Crump27
This technology was rapidly moved into the open-source community through the RepRap project Reference Jones, Haufe, Sells, Iravani, Olliver, Palmer and Bowyer28 and Fab@Home Reference Malone and Lipson29 and resulted in the cost of FDM-type 3D printers dropping from >USD$10,000 to <USD$1000 in less than a few years. The RepRap platform has since served as the foundation for many low-cost 3D bioprinters, where the thermoplastic extruder (hot end) has been replaced with a syringe-based extruder. Hinton et al. demonstrated that a USD$400 hobbyist-grade 3D printer could be modified into a functional 3D bioprinter Reference Hinton, Jallerat, Palchesko, Park, Grodzicki, Shue, Ramadan, Hudson and Feinberg11 and released the mechanical design for the syringe-based extruder (Figure 3b–c) using an open-source license through the National Institutes of Health 3D print exchange website. Reference Hinton30
Recently, startup companies have also emerged, such as CELLINK 31 and BioBots, 32 and started to produce much lower-cost 3D bioprinters with entry-level systems in the range of USD$10,000 (Figure 3d). This combination of low-cost open-source and commercial 3D bioprinters has expanded the number of research laboratories using these technologies to more than 100, and growing. Three-dimensional bioprinting encompasses more than just FDM and extrusion-based techniques, and recent patents that have expired in FDM, SLA, and SLS have expanded the range of low-cost AM processes accessible to biomaterials researchers.
Kinstlinger et al. demonstrated an open-source modification to a commercial laser cutter that incorporated powder handling to generate a low-cost SLS machine to work with poly(caprolactone), which is a low melting point thermoplastic biomaterial, for cell-adhesion studies (Figure 3e–f). Reference Kinstlinger, Bastian, Paulsen, Hwang, Ta, Yalacki, Schmidt and Miller33 There are many examples of research labs building or implementing low-cost 3D printing platforms for biomaterials research and tissue-engineering applications. Reference Hinton, Jallerat, Palchesko, Park, Grodzicki, Shue, Ramadan, Hudson and Feinberg11,Reference Kinstlinger, Bastian, Paulsen, Hwang, Ta, Yalacki, Schmidt and Miller33–Reference Dubbin, Hori, Lewis and Heilshorn36 As outlined in the articles in this MRS Bulletin issue, materials researchers are now leveraging the accessibility of these machines to focus on innovations in bioinks, support materials, and applications.
Multiscale integration and post-processing
Although human organs comprise dozens of cell types and multiple matrix types, the majority of bioprinting research currently utilizes only a single extruder nozzle and two to three cell types in the final engineered construct. It remains unclear as to what extent these engineered systems, when cultured, correctly reproduce the exquisite complexity found in the human body with the underlying assumption that better replication of architectures found in the body will lead to better physiologic function ( Figure 4 ). Reference Miller15,Reference Miller, Stevens, Yang, Baker, Nguyen, Cohen, Toro, Chen, Galie, Yu, Chaturvedi, Bhatia and Chen34,Reference Hubbell37 Thus, researchers are developing new generations of 3D printing capable of multihead or multimaterial fabrication. Reference Miller and Burdick14

Figure 4. Multiscale considerations for bioprinting. Organ-level functions (depicted here as the entire liver) can be studied at the meso- and microscales. The mesoscale comprises the “vascular unit cell,” which is a small functional unit of vascularized tissue with a patent vessel and supporting stromal and parenchymal organ-type cells that make up the rest of the organ-specific cells in the tissue. The microscale involves studying the phenotype of cells in the context of their immediate pericellular microenvironment where they receive and transmit chemical and biophysical cues and thereby provide function to the whole organism. These multiscale considerations can be studied through elaboration of principal architectural features, matrix and cellular components, and specific physiologic responses desired for study. Reference Miller15,Reference Miller, Stevens, Yang, Baker, Nguyen, Cohen, Toro, Chen, Galie, Yu, Chaturvedi, Bhatia and Chen34,Reference Hubbell37
Kang et al. developed an integrated four-head extrusion system capable of dispensing poly(caprolactone) threads, cellular inks, and sacrificial inks to make an integrated tissue-organ printer, which was utilized in studies of cartilage, mandibular bone, and skeletal muscle. Reference Kang, Jin Lee, Ko, Kengla, Yoo and Atala38 Lind et al. demonstrated cardiac tissue-on-a-chip integrated systems in which cardiac tissue was deposited alongside electrical sensors that could sense and measure the contractile performance of the tissue. Reference Lind, Busbee, Valentine, Pasqualini, Yuan, Yadid, Park, Kotikian, Nesmith, Campbell, Vlassak, Lewis and Parker39
Additional materials investigations have centered on post-processing to produce more complex scaffolds that cannot be produced by 3D printing. Jakus et al. demonstrated extrusion of hydroxyapatite mixed with poly(lactic-co-glycolic acid) and poly(caprolactone), and the resulting structures could be folded via origami to form new shapes that are not printable. Reference Jakus, Rutz, Jordan, Kannan, Mitchell, Yun, Koube, Yoo, Whiteley, Richter, Galiano, Hsu, Stock, Hsu and Shah40 The scaffolds were also able to promote bone growth in vitro, as well as in vivo in a rhesus macaque.
Further pushing materials boundaries, researchers are introducing “four-dimensional printing” (4D), in which the printed structure can self-assemble into a predetermined state based on changing environmental factors. Gladman et al. printed flat shapes that could self-assemble into flower-like morphologies through 3D printing of materials combinations, which could lead to anisotropic swelling.41 However, the types of tissue architectures that could be addressed by 4D printing have yet to be demonstrated.
The future
The integration of printed materials and cells, and the design of the proper architectures in which to place them are anticipated to be the primary focus over the next decade of bioprinting research. Scientists are determining the minimum feature set that is required in order to reconstitute organ-level function in controlled in vitro systems. Multidisciplinary teams will need to be formed composed not just of scientists, but also practicing clinicians who can advise on the final challenge of connecting engineered tissues directly to arteries and veins in a human patient. These exciting, burgenoning technologies and research communities promise to change the landscape of the medical industry and the ability to customize medical solutions for each individual patient.

Adam W. Feinberg is an associate professor in the Departments of Biomedical Engineering and Materials Science and Engineering at Carnegie Mellon University. He directs the Regenerative Biomaterials & Therapeutics Group, which develops materials-based engineering strategies to control the 3D self-organization and assembly of various cell types into ophthalmic, cardiovascular, and other tissue types. Feinberg received his BS degree in materials science and engineering from Cornell University in 1999, and his PhD degree in biomedical engineering from the University of Florida in 2004. Feinberg can be reached by phone at 412-268-4897 or by email at feinberg@andrew.cmu.edu.

Jordan S. Miller is an assistant professor of bioengineering at Rice University. He directs the Physiologic Systems Engineering and Advanced Materials Laboratory, which employs synthetic chemistry, open-source 3D printing, fluid dynamics, and molecular imaging to understand the process of vascularization and to assemble living tissues with complex organization. He received his BS degree in biology from the Massachusetts Institute of Technology in 2003, and his PhD degree in bioengineering from Rice University in 2008. Miller can be reached by phone at 713-348-8357 or by email at jmil@rice.edu.