Converting the surfaces of lead halide perovskites to water-insoluble lead oxysalt can significantly stabilize perovskite solar cells, a team led by Shuang Yang, Shangshang Chen, and Jinsong Huang of the University of North Carolina at Chapel Hill reported in a recent issue of Science (doi:10.1126/science.aax3294).
Previous approaches have focused on stabilizing perovskites themselves or by physically covering perovskites with organic molecules. “The secondary bonding between passivation molecules and the perovskite surface is typically too weak to protect perovskites from attack by moisture and oxygen,” Huang says. By contrast, the new approach involves forming a compact, strongly bonded lead oxysalt layer through a reaction of the perovskites with sulfate or phosphate ions.
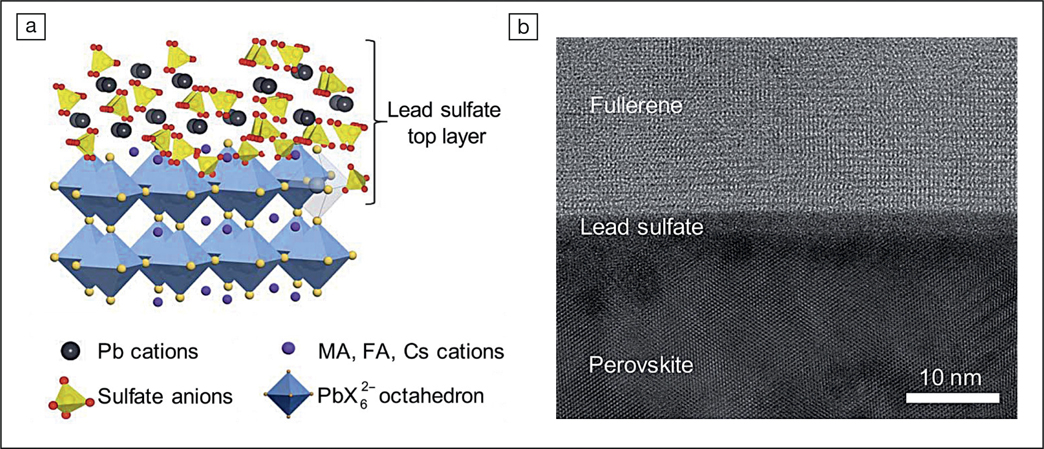
(a) Schematic illustration of protection of perovskites through in situ formation of a lead sulfate top layer on the perovskite surface. (b) Cross-sectional high-resolution transmission electron microscope image of the perovskite/lead sulfate/fullerene interface (fullerene is the charge-transport layer). Credit: Science.
The oxysalt capping protects the perovskite and suppresses defects at the surface. Encapsulated solar cells made with the capped perovskites maintained about 97% of their initial efficiency with simulated solar irradiation for nearly two months at a realistic operation temperature of 65°C. “This is one of the best reported stability so far, if not the best,” Huang says.
Astudy in the journal Materials Horizons offers an insight into the low defect density of halide perovskites. This could help materials scientists search for and design materials with similarly low defect densities for applications in optoelectronics and beyond (doi:10.1039/c9mh00606k).
Defects in semiconductors, including halide perovskites, drastically impact the materials’ electronic and optical properties. Perovskites typically have very few defects especially when solution-processed near room temperature. David Cahen of the Weizmann Institute of Science and his colleagues had previously found that defects in hal-ide perovskites can repair themselves because of dynamic bonds that can break and reform. “This implies change toward a thermodynamically more stable state,” Cahen says.
These findings, he says, led the researchers to propose the counter-intuitive idea that “the materials’ very small free energy of formation from their binary components, which stabilizes them against decomposition, leads to their ultra-low densities of static defects,” Cahen says. This understanding suggests a new path to defect management in materials, the research team concluded.
Perovskite solar cells are prone to degradation when exposed to humidity and sunlight, and researchers have been devising various ingenuous ways to improve their stability. A University of Oxford research team led by Sai Bai, Feng Gao, and Henry Snaith have found that adding ionic liquids to perovskites markedly improves the devices’ long-term stability.
The researchers added the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate to the formamidinium-methylammonium-cesium lead hal-ide perovskite. They made a solar cell by using this light-absorbing layer between electron and hole extraction layers. The most stable encapsulated devices lost just 5% of their roughly 20% power-conversion efficiency after being exposed to simulated sunlight for more than 1800 hours at 70–75°C. The team estimates that device efficiency drops to 80% of its peak performance in about 5200 hours.
Ions migrate in the perovskite layer, especially under light and heat, creating defects that can trap charges and bring down efficiency. The ionic liquid unpredictably suppressed this ion migration, and it also facilitates better charge transfer between the perovskite and charge-transport layer, the team reported in a recent issue of Nature (doi:10.1038/s41586-019-1357-2).
Researchers at Kyushu University in Japan have made exceptionally thick organic light-emitting diodes (OLEDs) by combining thin organic light-emitting films with hybrid perovskite charge-transport layers. They published their results in Nature (doi:10.1038/s41586-019-1435-5).
OLEDs hold promise for low-cost, flexible displays and lighting. They are made of a layer of organic light-emitting molecules sandwiched between organic charge-transport layers. The transport layers need to be thick to completely cover the defects and residues on a substrate. But this requires high driving voltage because organic materials are usually poor conductors.
Toshinori Matsushima, Chihaya Adachi, and their colleagues used the perovskite methylammonium lead chloride to make transport layers that were 2000-nm thick, more than 10 times the thickness of standard OLEDs, without requiring high driving voltage. The thick OLEDs could be used to make large, low-cost, efficient displays that emit the same color from different viewing angles.


