Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Jang, Y.-I.
Huang, B.
Wang, H.
Chiang, Y.-M.
and
Sadoway, D. R.
1997.
Effects of Aluminum Doping on The Phase Stability and Electrochemical Properties of LiCoO2 and LiMnO2.
MRS Proceedings,
Vol. 496,
Issue. ,
Julien, C.
Massot, M.
Perez-Vicente, C.
Haro-Poniatowski, E.
Nazri, G. A.
and
Rougier, A.
1997.
Vibrational Spectroscopic Studies of the Local Environment in 4-Volt Cathode Materials.
MRS Proceedings,
Vol. 496,
Issue. ,
Julien, C.
and
Nazri, G.A.
1998.
General Overview of Vibrational Spectroscopy of Layered Transition-Metal Oxides.
MRS Proceedings,
Vol. 548,
Issue. ,
Jang, Young-Il
Huang, Biying
Wang, Haifeng
Maskaly, Garry R
Ceder, Gerbrand
Sadoway, Donald R
Chiang, Yet-Ming
Liu, Hui
and
Tamura, Hirokazu
1999.
Synthesis and characterization of LiAlyCo1−yO2 and LiAlyNi1−yO2.
Journal of Power Sources,
Vol. 81-82,
Issue. ,
p.
589.
Julien, C.
Michael, S.S.
and
Ziolkiewicz, S.
1999.
Structural and electrochemical properties of LiNi0.3Co0.7O2 synthesized by different low-temperature techniques.
International Journal of Inorganic Materials,
Vol. 1,
Issue. 1,
p.
29.
Julien, C.
1999.
Study of the local order in LiNi1−yCoyO2 oxides synthesized by soft chemistry.
Ionics,
Vol. 5,
Issue. 5-6,
p.
351.
Julien, C.
2000.
Materials for Lithium-Ion Batteries.
p.
309.
Julien, C.
2000.
Structure, morphology and electrochemistry of doped lithium cobalt oxides.
Ionics,
Vol. 6,
Issue. 5-6,
p.
451.
Julien, C.
2000.
Materials for Lithium-Ion Batteries.
p.
223.
Julien, C
Camacho-Lopez, M.A
Lemal, M
and
Ziolkiewicz, S
2002.
LiCo1−yMyO2 positive electrodes for rechargeable lithium batteries.
Materials Science and Engineering: B,
Vol. 95,
Issue. 1,
p.
6.
Castro-Couceiro, A.
Castro-García, S.
Señarís-Rodríguez, M. A.
Soulette, F.
and
Julien, C.
2002.
Effect of the aluminium doping on the microstructure and morphology of LiNi0.5Co0.5O2 oxides.
Ionics,
Vol. 8,
Issue. 3-4,
p.
192.
Amdouni, N
Zarrouk, H
Soulette, F
and
Julien, C
2003.
LiAlyCo1−yO2 (0.0≤y≤0.3) intercalation compounds synthesized from the citrate precursors.
Materials Chemistry and Physics,
Vol. 80,
Issue. 1,
p.
205.
Amdouni, N.
Zarrouk, H.
and
Julien, C. M.
2003.
Structural and electrochemical properties of LiCoO2 and LiAlyCo1−yO2 (y=0.1 and 0.2) oxides: A comparative study of electrodes prepared by the citrate precursor route.
Ionics,
Vol. 9,
Issue. 1-2,
p.
47.
Amdouni, N.
Zarrouk, H.
and
Julien, C.
2003.
Low temperature synthesis of LiCr0·3Co0·7O2intercalation compounds using citrate, oxalate, succinate, and glycinate precursors.
British Ceramic Transactions,
Vol. 102,
Issue. 1,
p.
27.
Amriou, T.
Sayede, A.
Khelifa, B.
Mathieu, C.
and
Aourag, H.
2004.
Effect of Al-doping on lithium nickel oxides.
Journal of Power Sources,
Vol. 130,
Issue. 1-2,
p.
213.
Ganesan, M.
Sundararajan, S.
Dhananjeyan, M.V.T.
Sarangapani, K.B.
and
Renganathan, N.G.
2006.
Synthesis and characterization of tetravalent titanium (Ti4+) substituted LiCoO2 for lithium-ion batteries.
Materials Science and Engineering: B,
Vol. 131,
Issue. 1-3,
p.
203.
Julien, C.M.
Mauger, A.
Groult, H.
Zhang, X.
and
Gendron, F.
2011.
LiCo1−yByO2 As Cathode Materials for Rechargeable Lithium Batteries.
Chemistry of Materials,
Vol. 23,
Issue. 2,
p.
208.
Zhang, X.
Julien, C.M.
Mauger, A.
and
Gendron, F.
2011.
Magnetic analysis of lamellar oxides for Li-ions batteries.
Solid State Ionics,
Vol. 188,
Issue. 1,
p.
148.
Manthiram, Arumugam
Knight, James C.
Myung, Seung‐Taek
Oh, Seung‐Min
and
Sun, Yang‐Kook
2016.
Nickel‐Rich and Lithium‐Rich Layered Oxide Cathodes: Progress and Perspectives.
Advanced Energy Materials,
Vol. 6,
Issue. 1,
刘, 华东
2020.
Study on the Influence of Impurity Iron in Mn<sub>3</sub>O<sub>4</sub> Manganese Source on the Electrical Properties of LiMn<sub>2</sub>O<sub>4</sub> Positive Electrode Material.
Material Sciences,
Vol. 10,
Issue. 09,
p.
759.
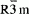 space group. The IR spectra of the samples indicate formation of compressed CoO6 and NiO6, and elongated LiO6 octahedra. The IR vibrational mode of the LiO6 remains in the 200–300 cm-1 and the vibrational modes of the MO6 expand over 400–650 cm-1. Results of long charge-discharge cycling of the samples as cathode materials in lithium cells showed long cycle life. The capacity of the electrodes upon substitution were reduced almost linearly as the concentration of substitution was increased. The solubility limit for the formation of solid solutions upon substitution of Al and B in LiNiO2 and LiCoO2 was found to be around 25%. Specific capacities of the samples were between 120 to 160 mAh/g depending on the amount of substitution.
space group. The IR spectra of the samples indicate formation of compressed CoO6 and NiO6, and elongated LiO6 octahedra. The IR vibrational mode of the LiO6 remains in the 200–300 cm-1 and the vibrational modes of the MO6 expand over 400–650 cm-1. Results of long charge-discharge cycling of the samples as cathode materials in lithium cells showed long cycle life. The capacity of the electrodes upon substitution were reduced almost linearly as the concentration of substitution was increased. The solubility limit for the formation of solid solutions upon substitution of Al and B in LiNiO2 and LiCoO2 was found to be around 25%. Specific capacities of the samples were between 120 to 160 mAh/g depending on the amount of substitution.