Article contents
Structure determination of two structural analogs, named 3-[1-(2-fluoro-4-biphenyl)ethyl]-6-(4-fluorophenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (C23H16F2N4S) and 3-[1-(2-fluoro-4-biphenyl)ethyl]-6-(4-chlorophenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (C23H16ClFN4S) by synchrotron X-ray powder diffraction
Published online by Cambridge University Press: 19 December 2017
Abstract
Two novel compounds, 3-[1-(2-fluoro-4-biphenyl)ethyl]-6-(4-fluorophenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (C23H16F2N4S) (1) and 3-[1-(2-fluoro-4-biphenyl)ethyl]-6-(4-chlorophenyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole (C23H16ClFN4S) (2), have been designed and synthesized as cytotoxic agents. The compounds were characterized by infrared, proton nuclear magnetic resonance, mass spectral data, elemental analysis and X-ray powder diffraction. The present study comprises spectral data and crystal structures of these novel compounds determined from synchrotron X-ray powder diffraction data. The structure solutions were obtained by simulated annealing. The final structures were achieved by Rietveld refinement using soft restraints for all bond lengths, bond angles, and planar groups. Both compounds crystallize in space group 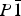 $P\bar 1$ , Z = 2, with the unit-cell parameters a = 6.37433(9), b = 11.3641(2), c = 14.09115(19) Å, α = 80.1740(8)°, β = 85.1164(8)°, γ = 80.9831(10)°, V = 991.55(3) Å3 of compound (1) and a = 6.53736(6), b = 11.55725(15), c = 14.01373(13) Å, α = 80.3323(7)°, β = 84.8939(6)°, γ = 79.3954(8)°, V = 1024.08(2) Å3 of compound (2). Structural analyses reveal that the title compounds are isostructural.
$P\bar 1$ , Z = 2, with the unit-cell parameters a = 6.37433(9), b = 11.3641(2), c = 14.09115(19) Å, α = 80.1740(8)°, β = 85.1164(8)°, γ = 80.9831(10)°, V = 991.55(3) Å3 of compound (1) and a = 6.53736(6), b = 11.55725(15), c = 14.01373(13) Å, α = 80.3323(7)°, β = 84.8939(6)°, γ = 79.3954(8)°, V = 1024.08(2) Å3 of compound (2). Structural analyses reveal that the title compounds are isostructural.
Keywords
Information
- Type
- New Diffraction Data
- Information
- Copyright
- Copyright © International Centre for Diffraction Data 2017
References
- 2
- Cited by

