Introduction
Since the Aum Shinrikyo sarin nerve agent attacks in 1994 and 1995, respectively, civilian populations have been the target of chemical attacks. Reference Sidell, Patrick and Dashiell1–Reference Okumura, Suzuki and Fukuda7 In their study, Ruckart, et al listed approximately 50 industrial chemicals that have the potential to be used in a terrorist plot against civilian populations. Reference Ruckart and Fay8 This, in conjunction with the existing threat posed by chemical warfare agents reported to act within seconds to hours, Reference Schulz-Kirchrath9–Reference Schwenk15 stresses the requirement to develop a medical preparedness capability. Reference Baker16–Reference Treat, William and Furbee21 In the literature, there is a lack of medical guidelines and protocols for prehospital management in conjunction with the integrated use of protection and decontamination capabilities for both the health care professionals and the patients in the event of a chemical attack or other types of exposure (eg, biological, radiological, and nuclear). 10,22–30 Furthermore, little is still known regarding the clinical impact of chemical exposures in humans. These knowledge gaps expose any population to inappropriate clinical care in the eventuality of a chemical attack with (or without) mass casualties, and therefore, a higher risk of death or long-term disability. Reference Mukaida, Hattori and Iwamoto31,Reference Nishimoto, Burrows and Miyanishi32
The aim of this systematic review was to investigate the clinical knowledge and evidence-based practices applied in patients exposed to chemical weapons and treated in a prehospital or acute setting in order to identify the knowledge gaps that related to an efficient mass-casualty management in a contaminated environment. Ultimately, the objective was to compare the clinical outcomes of patients exposed to a chemical attack who received known interventions to reduce the risk of further contamination and progression of the harmful effects of the chemical (ie, protection, decontamination, and treatment).
Methods
Study Design
This study is a systematic review of the literature. The recommendations of the Cochrane Handbook for Systematic Reviews of Interventions were followed. 33 The protocol was registered in the international register for systematic reviews maintained by the National Institute for Health Research (United Kingdom; PROSPERO Registration Number: CRD42019104473, Accepted on February 25, 2019; https://www.crd.york.ac.uk/prospero/; Last Update November 24, 2020).
Source of Data
Online databases used for this study were: MEDLINE (US National Library of Medicine, National Institutes of Health; Bethesda, Maryland USA); Web of Science Core Collection (Thomson Reuters; New York, New York USA); Embase (Elsevier; Amsterdam, Netherlands); Cochrane (The Cochrane Collaboration; London, United Kingdom); and CINAHL (EBSCO Information Services; Ipswich, Massachusetts USA) from their inception through November 6, 2018. An update was performed on September 16, 2020 (Supplementary Material, Table S1; available online only).
Search Strategy
Indexed and free-text terms, such as Respiratory, Warfare, and Chemical Threat, were selected by individually combining each of the two warfare modes with respiratory distress (Supplementary Material, Table S1). Afterwards, references were imported into the Covidence systematic review software (Veritas Health Innovation; Melbourne, Australia). Duplicate papers were automatically rejected by this software. Pre-trained individuals performed an abstract triage trial run on 40 selected references. Titles and abstracts were then independently screened by two reviewers and were retained for a full-text review if they met the inclusion/exclusion criteria listed in the next paragraph. Full texts of selected abstracts were then retrieved and assessed by two reviewers to confirm eligibility. At any point in the above-mentioned process, disagreements between reviewers were resolved using a consensus approach.
Inclusion and Exclusion Criteria
Inclusion criteria were: (1) exposure to a chemical incident (eg, mass casualties); (2) chemical known to affect the respiratory system; (3) interventions involving the assessment of a triad of integrated key competences (protection for staff and patients, decontamination, and treatments); (4) patient outcomes (ie, primary: patient’s health condition remaining stable due to medical, protection, and decontamination interventions; secondary: patient’s mortality occurring at his/her admission despite medical, protection, and decontamination interventions); (5) studies with original data, including those conducted on animals induced with chemical agents in order to simulate a medical extraction of casualties; and (6) studies should have occurred within the zone of interest. The zone of interest where medical interventions took place in eligible studies was defined as the casualty extraction from the incident site where the chemical attack occurred to the clean zone where the patient was admitted to the hospital (Figure 1).

Figure 1. Illustration of the Field of Clinical Practice in Acute or Prehospital Settings in Contaminated Environments.
Note: This is a summary of the zone of interest of this study (ie, from the incident site to the transfer of the patient in a clean zone, after being transported through the contamination environment, and then fully decontaminated). During a medical extraction from the contaminated environment (ie, hot and warm zones), the ideal mitigation measure against contaminants is facing upwind. Ideally, a very light decontamination process, called immediate decontamination, will be performed immediately after an attack/exposure to slow the agent’s absorption into the body. Thorough decontamination is a specialized process that occurs later, ideally prior to admission to a medical facility. Number 1 – Clinical process occurring from the moment the patient is handled until decontamination is completed; Number 2 – Continuity of care happening at the patient’s transfer, admission, and beyond within a medical facility (eg, emergency room or intensive care unit).
Studies were excluded if: (1) effects were shown on insects, plants, or materials; (2) procedures were performed in a clean/cold zone setting once the patient was fully admitted and handled by the medical facility’s staff; (3) they did not address a respiratory disorder; (4) they did not present original data (eg, reviews); or (5) the topic was not related to a chemical threat (eg, suicide attempt).
Quality Appraisal/Risk of Bias
Two quality appraisal charts were used in order to detect and mitigate the variability in staffers’ assessments. The first was developed by Hong, et al from McGill University (Montreal, Quebec, Canada; Reference Hong, Pluye and Fàbregues34 the second was from Hawker, et al (Appendices C and D from that research). Reference Hawker, Payne and Kerr35 The risk of bias in each eligible study was assessed independently by two reviewers.
Extraction of Data
Data extraction was performed independently by two individuals. Extracted data were imported into an Excel (Microsoft Corp.; Redmond, Washington USA) spreadsheet format developed in-house based on Cochrane and Covidence models (Supplementary Material, Table S2; available online only).
Synthesis of Evidence
The method for qualitative synthesis of evidence that was used led to produce different summaries: (1) health management plan and clinical tools used to respond to a chemical attack; (2) detection of toxidromes in the patient’s condition versus the clinical intervention provided; (3) delays to response; and (4) association between these variables. Further details are found in the Supplementary Material (Body Text; available online only).
Biostatistical Analysis
Descriptive statistics were planned to summarize study characteristics, including mean and standard deviations (SD) and median and interquartile range (IQR) and proportions, according to the type of data. A Student’s t-test was planned to compare the clinical onset of chemical agents and algorithms of treatment, along with forest plots to highlight the difference between each agent’s action mechanism and therapy onsets. To mitigate the potential impact of missing data, an imputation model was planned (root mean square error). Descriptive statistics and other numbers were to be computed with IBM SPSS Statistics Software (SPSS Inc.; Chicago, Illinois USA) and StatsDirect statistical software (StatsDirect Ltd.; Sale, Cheshire, United Kingdom). A meta-analysis involving the use of a random effects linear model (mixed effects model) was planned to correlate the effect of a studied chemical agent with one of the clinical interventions made by health care professionals. This would have highlighted the windows of treatment opportunities in such contaminated environments (RevMan software version 5.3; The Cochrane Collaboration Network; London, United Kingdom). The statistical significance level was set at P <.05 and interpreted with 95% confidence intervals (CI). These biostatistics plans had been reviewed by a biostatistician. Unfortunately, it was not possible to run any statistical analysis due to the heterogeneity of eligible studies and paucity of extractable data.
Results
The flowchart Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram is presented in Figure 2. A PRISMA checklist was also used based on the Prehospital and Disaster Medicine journal’s instruction for authors 36 (Supplementary Material, Table S3; available online only). After title and abstract screening, 969 of the 1,641 studies identified through the search strategy remained eligible for full-text assessment. In the end, only four studies (all related to a sarin gas attack) were eligible for inclusion in this systematic review. Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 No further studies were added after the update performed in September 2020.

Figure 2. PRISMA Diagram.
Abbreviations: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ICU, intensive care unit.
Eligible studies Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 reported retrospective data on patients or health care professionals treated in a contaminated cold zone (eg, medical facilities) and contained some information related to the acute settings (Figure 1). The patients included in these four studies were victims of three different events: (1) the 1994 Matsumoto suburban terrorist attack (Japan); Reference Yanagisawa, Morita and Nakajima40 (2) the 1995 Tokyo subway terrorist attack (Japan); Reference Nozaki, Hurl and Shinozawa37,Reference Okumura, Takasu and Ishimatsu38,Reference Yanagisawa, Morita and Nakajima40 and (3) the 2013 Damascus civil war attack (Syria). Reference Rosman, Eisenkraft and Milk39 In the case of the Tokyo attack, one paper reported some data Reference Yanagisawa, Morita and Nakajima40 that were present in two others. Reference Nozaki, Hurl and Shinozawa37,Reference Okumura, Takasu and Ishimatsu38 In this analysis, information was considered common to two or more of the studies as a single data set. In other words, matching results were treated as a single response, but when different results were presented, these accounted for two independent medical responses and are reported as such in this paper.
Quality Appraisals
The quality appraisals are presented in Table S4 and Table S5 (Supplementary Material; available online only) of the supplement. Overall, eligible studies showed a moderate to high risk of bias. The two tools used provided similar results.
Subjects Characteristics and Outcomes
A summary of each study is presented in Table 1. Based on the limited available data scattered throughout the eligible studies, Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 this study estimates that a minimum of 8,550 individuals were exposed during the sarin gas attacks that struck Japan and Syria, of which 1,333 casualties and 11 deaths were confirmed medical cases managed on the day the attacks occurred (Supplementary Material, Table S6 and Table S7; available online only). The 1,333 casualties represented confirmed chemical poisoning cases in acute settings and were considered in this study as the number of included patients.
Table 1. Summary of Included Studies

Abbreviations: CPR, cardiopulmonary resuscitation; CWA, chemical warfare agents; ED, emergency departments; M, Matsumoto; T, Tokyo.
* Unawareness of a CWA attack.
∫ – Design deduced from the paper as authors did not specify their design.
£ – The source of data is deduced from the paper as it was not provided by the authors.
† – Measurement not substantiated in the literature.
‡ – No biostatistics plan and analysis.
! – This represents the minimum number of patients managed by medical authorities over the years above the numbers treated in acute settings and reported in this paper.
€ – Secondary exposures confirmed by authors (ie, expansion of the contamination zone due to contaminated carriers [casualty/vehicle]).
§ – Signs of secondary exposures (ie, issues with PPE and decontamination capabilities, health care staff, and other rescuers becoming sick or absence of specialized capabilities).
¥ – Visual Analogue Scale Grade (No information confirmed – Absence of information about the topic/category confirmed; Not reported – Uncertainty as to whether the authors might or might not have analyzed this topic/category; Partially – little information available; Detail(s) provided – Disclosure of the information).
Overview of the Populations Treated in Acute Settings
Nozaki, et al was the only study to provide a complete basic breakdown of the affected population (n = 15 medical staff members; 13 males, two females, all Japanese, ages ranging from 25 to 51 years old). Reference Nozaki, Hurl and Shinozawa37 In the Okumura, et al study, 640 patients were treated but the authors only provided a partial breakdown (395 males, five pregnant females; aged eight to 65 years old). Reference Okumura, Takasu and Ishimatsu38 No information was provided on the remaining 240 individuals poisoned. Reference Okumura, Takasu and Ishimatsu38 In their study, Yanagisawa, et al reported that the 1994 Matsumoto attack resulted in a total of seven fatalities and 272 casualties treated the day of the attack (264 patients; eight rescuers). Reference Yanagisawa, Morita and Nakajima40 In the 1995 Tokyo attack, the same authors reported four dead and 920 survivors treated the day of the attack: (1) St. Luke’s Hospital: 750 (one dead; 749 affected individuals, 639 patients and 110 medical staff members); (2) Keio University: one dead, 85 patients, 15 medical members; (3) Teishin Hospital: 32 patients and 39 rescuers; and (4) Tokyo Subway Station: two dead. Reference Yanagisawa, Morita and Nakajima40 Age and gender were not reported. Reference Yanagisawa, Morita and Nakajima40 Rosman, et al provided a casualty estimate (n = 130; 3% females, 97% males; of which 60% were children) based on their source of data (YouTube [Google, Inc.; San Bruno, California USA] social media footage analysis). Reference Rosman, Eisenkraft and Milk39
Medical Interventions During Casualty Extraction
None of the four papers Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 provided comprehensive details regarding treatments given to patients as a function of symptomatology during the medical extraction. In the Nozaki, et al study, no details were provided regarding the medical interventions performed on the 85 contaminated patients upon arrival at Keio University Hospital. In addition, their clinical presentation during the medical extraction from the chemical attack site in Tokyo and once admitted to hospital was not reported by the authors. Reference Nozaki, Hurl and Shinozawa37 However, the authors did report some information on two patients’ respective health conditions, one convulsive and one in cardiac arrest, for which cardiopulmonary resuscitation (CPR) was performed upon the transfer to the emergency department (ED)/emergency room (ER). Reference Nozaki, Hurl and Shinozawa37 Table 2 lists the available information related to the continuity of care provided by the Japanese medical centers at the patient’s admission.
Table 2. Listed Treatments Patients Received Once Admitted
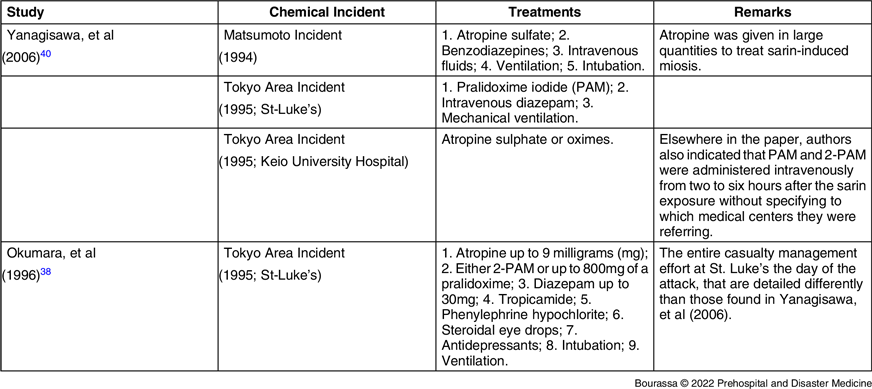
Note: There was no indication on the use of oxygen found in these studies.
Abbreviations: PAM, pralidoxime iodide; 2-PAM, 2-pyridinealdoxime methiodide.
In Okumara, et al, one person performed CPR on a victim at the site of the chemical attack in Tokyo before getting poisoned by sarin herself. At her arrival at St. Luke’s ER with two other victims, that Samaritan also was in cardiac arrest. Reference Okumura, Takasu and Ishimatsu38 Regarding medical extraction, the authors only reported the transportation performed by paramedics. Reference Okumura, Takasu and Ishimatsu38 No information was provided on the medical interventions performed on 99 patients during their transport to hospital by first responders. Reference Okumura, Takasu and Ishimatsu38 Similarly, the authors did not report on first aid performed by good Samaritans or health care staff for the remaining 541 rescued patients. Reference Okumura, Takasu and Ishimatsu38
Yanagisawa, et al reported that all cardiac arrest patients from Matsumoto (1994; n = 3) and Tokyo (1995; n = 5) were treated upon arrival to the ED, but no further detail was provided. Reference Yanagisawa, Morita and Nakajima40
In Rosman, et al, the authors listed treatments provided to patients in non-medical facilities: atropine, steroids, furosemide, supplemental oxygen (O2), nasopharyngeal suctioning, bag valve ventilation, tracheal intubation, mechanical ventilation, and chest compression. Reference Rosman, Eisenkraft and Milk39 They noted that standard monitoring equipment was not used to measure O2 saturation, blood pressure, or cardiac electrical activity. Reference Rosman, Eisenkraft and Milk39 As noted by the authors, all medication was administered intravenously with no evidence of autoinjector use. Reference Rosman, Eisenkraft and Milk39 Moreover, they casted doubt on the authenticity of 66 out of 67 YouTube videos analyzed.
The two studies of the 1995 incidents that occurred in Japan Reference Okumura, Takasu and Ishimatsu38,Reference Yanagisawa, Morita and Nakajima40 did not report whether the delivery procedures or special gestational care successfully preserved the life of the fetus/newborn or not. Similarly in the Damascus incident, Rosman, et al only mentioned that children accounted for 60% of the 130 casualties. Reference Rosman, Eisenkraft and Milk39 Okumara, et al reported an eight-year-old victim as the youngest casualty treated at St. Luke’s Hospital, but no further clinical information was provided. Reference Okumura, Takasu and Ishimatsu38 Likewise, no information was reported for specific populations.
Medical Interventions Due to Secondary Exposure in Rescuers and Medical Staff
Nozaki, et al was the only study Reference Nozaki, Hurl and Shinozawa37 of the four Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 to have reported a medical response involving medical staff affected by managing patients contaminated by sarin, which led to a secondary exposure or the relocation of the contaminated zone to an unprepared location. The authors reported a six-hour wait before medical staff received confirmation that sarin gas was the cause of patient intoxications. Reference Nozaki, Hurl and Shinozawa37 In the interim, medical staff provided medical care for an unknown exposure. Reference Nozaki, Hurl and Shinozawa37 The authors briefly described some close-contact events that occurred between 15 clinicians and 85 contaminated patients. Reference Nozaki, Hurl and Shinozawa37 Of these cases, only two were summarily described (the medical management of one convulsive and one cardiac arrest case). The authors also enumerated 14 of 15 reported total cases involving medical staff according to the following categories: four cardiac arrests, two intubations, three cases of contamination, four unspecified tasks, and one observation. Of these, six adult caregivers received atropine (0.5-1.0mg intramuscular); one caregiver received 2-PAM (500mg). Reference Nozaki, Hurl and Shinozawa37 Other causative factors that led to a secondary exposure are covered in the Results section and the Supplementary Material (Body Text; available online only).
Medical Algorithms
Throughout the four studies, Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 there was no indication that specific chemical intoxication algorithms or clinical guidelines for patient management were used, except for triage. Reference Nozaki, Hurl and Shinozawa37,Reference Rosman, Eisenkraft and Milk39 Medical authorities in Matsumoto Reference Yanagisawa, Morita and Nakajima40 (1994) and medical staff at St. Luke’s Hospital (1995) used an algorithm Reference Okumura, Takasu and Ishimatsu38 for patients’ triage (mild, moderate, and severe) and management, Reference Okumura, Takasu and Ishimatsu38,Reference Yanagisawa, Morita and Nakajima40 even if the exact terms used varied. It should be noted that even though the two studies partly covered the same medical response at St. Luke’s Hospital, Reference Okumura, Takasu and Ishimatsu38,Reference Yanagisawa, Morita and Nakajima40 their respective authors did not report precisely the same version of the triage score. No gold-standard reference related to that triage score was found in either study. Reference Okumura, Takasu and Ishimatsu38,Reference Yanagisawa, Morita and Nakajima40 The definitions are shown in Table S8 of the Supplementary Material (available online only).
Immediate and Specialized Decontamination Capabilities
None of the four papers Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 reported whether or not immediate decontamination procedures were performed during the extraction process before their arrival at a specialized decontamination asset before admission to a medical facility, usually considered as a clean zone. None of the papers reported the existence of specialized assets capable of combining actions like continuing medical treatments, performing decontamination, and ensuring safety while wearing personal protective equipment (PPE). Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40
However, three papers Reference Nozaki, Hurl and Shinozawa37–Reference Rosman, Eisenkraft and Milk39 out of the four Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 provided details on some of the decontamination means used on patients while at the medical facilities. Nozaki, et al reported: (1) ventilation of resuscitation rooms was ensured by opened doors and windows; and (2) contaminated belongings were placed in sealed vinyl bags. Reference Nozaki, Hurl and Shinozawa37
Okumara, et al summarized decontamination steps as the removal of contaminated clothing. Reference Okumura, Takasu and Ishimatsu38 They also reported that patients were either showered or bathed depending on their state of consciousness, but provided no further detail. Reference Okumura, Takasu and Ishimatsu38 In the case of Rosman, et al, their observation of procedures was reported as: (1) wash out with water, which included rubbing the casualty’s face and chest (25% of videos); and (2) full removal of clothing (10 out of 67 videos in which decontamination took place at medical facilities with no additional information provided). Reference Rosman, Eisenkraft and Milk39
Personal Protective Equipment
None of the four studies confirmed PPE was worn during the management of the chemical attacks. Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 Rather, while two studies made no mention of PPE for rescuers, the clinicians, and the patients, Reference Nozaki, Hurl and Shinozawa37,Reference Okumura, Takasu and Ishimatsu38 the two remaining studies presented little information on their means of protection. Reference Rosman, Eisenkraft and Milk39,Reference Yanagisawa, Morita and Nakajima40 The authors reported that health care professionals were not protected from contamination despite the suspicion of gas poisoning. Reference Yanagisawa, Morita and Nakajima40 Regarding the Tokyo attack, the authors confirmed rescue staff did not use special PPE to protect themselves against the gas exposure. Reference Yanagisawa, Morita and Nakajima40 They neither specified the members of the rescue teams nor if the medical staff used any PPE. Reference Yanagisawa, Morita and Nakajima40 In Rosman, et al, it was reported that no PPE was worn other than the sporadic use of latex gloves and surgical masks by medical staff (10 out of 67 videos). Reference Rosman, Eisenkraft and Milk39
Other Causes of Secondary Exposures
As previously indicated, Nozaki, et al Reference Nozaki, Hurl and Shinozawa37 was the only study of the four Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 that covered the medical management of new patients (ie, medical staff) due to secondary exposures induced by contaminated patients. For its part, Yanagisawa, et al identified failures in PPE capabilities as a cause of secondary exposure to rescuers for the Matsumoto and Tokyo chemical attacks. Reference Yanagisawa, Morita and Nakajima40
Meta-Analysis
Due to the paucity of studies and the heterogeneity of the data, no meta-analysis was performed.
Discussion
Major Findings
In this systematic review, results showed that very few studies reporting on acute medical care after a chemical terrorist attack or civil war clash have been published so far. Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 No clinical data were found regarding mass-casualty management from the incident site to the point of transfer at a medical facility (ie, acute settings). According to available information, the treatments delivered to victims were very heterogeneous and no dedicated algorithm was used. Also, there were major protection and decontamination capability deficiencies (eg, standardization, equipment, and their application in medical interventions) for both patients and staff. These led not only to secondary contamination of health care professionals and medical facility environments, but may also have played a role in the worsening of patients’ conditions.
One study identified by the search strategy concerned the 2014 chemical attack in Syria, Reference Rosman, Eisenkraft and Milk39 while the remaining three Reference Nozaki, Hurl and Shinozawa37,Reference Okumura, Takasu and Ishimatsu38,Reference Yanagisawa, Morita and Nakajima40 addressed the 1994 and 1995 events in Matsumoto and Tokyo, respectively. Lack of detail regarding medical interventions reported by the authors Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 hindered the ability to assess the adequacy of the interventions performed on patients as no mention was made of gold standards, guidelines, or protocols. In some instances, only resuscitation maneuvers were reported, Reference Nozaki, Hurl and Shinozawa37,Reference Okumura, Takasu and Ishimatsu38,Reference Yanagisawa, Morita and Nakajima40 and no information were provided regarding PPE and decontamination capabilities for patients, rescuers, or health care professionals. Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40
Due to the modest quantity and quality of the studies identified by the search strategy and the heterogeneity of the data, researchers were unable to proceed with the biostatistical analysis plan. This situation was not precedent-setting as in McGaughey, et al, a systematic review conducted on an early-warning system, experienced similar challenges with two included studies (ie, showing poor evidence, impossible to make comparisons). Reference McGaughey, Alderdice and Fowler41
Importance of Medical Algorithms, Treatment Capabilities, and Disaster Plan
With the exception of three studies which showed that similar triage systems were used in the management of casualties during the chemical attacks in Japan, Reference Okumura, Takasu and Ishimatsu38–Reference Yanagisawa, Morita and Nakajima40 the use of a medical algorithm or a clinical guideline was not reported in selected studies. Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 It should also be noted that Okumura, et al reported triage categories using terms more directly related to the clinical response, Reference Okumura, Takasu and Ishimatsu38 while Yanagisawa, et al simply listed the definitions with barely any clinical detail regarding the events in Matsumoto and at St. Luke’s hospital in Tokyo. Reference Yanagisawa, Morita and Nakajima40
Only one study mentioned the activation of the disaster plan at St. Luke’s Hospital, Reference Okumura, Takasu and Ishimatsu38 which also strengthens the argument concerning a complete lack of preparedness to deal with such disasters. Most importantly, the means of treatment and the overall capability during the medical extraction of patients from the incident site to their transfer to the ER, presumably after a thorough decontamination, was not reported. Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 The decontamination aspect is of particular importance in situations where secondary exposures occurred in rescuers and medical staff at unprepared locations. Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 At first glance, this suggests that algorithms for clinical response in acute settings or during an extraction within a contaminated environment need to be further developed, more widely disseminated, and regularly updated. However, the passage of time between the attacks, the publication of the related studies, and present-day knowledge and recommendations available in the grey medical literature of several organizations render comparisons fruitless. This nonetheless also suggests that recommended medical practices should, on the one hand, be subjected to more scrutiny in order to integrate medical developments and innovations such as O2 therapy, and should also, on the other hand, focus on the application of novel technologies in the acute settings field of research, including capabilities offered by artificial intelligence. Thus, this could be envisioned as a research study in itself, or even an entire research program.
Importance of Protection and Decontamination Capabilities
Throughout the four papers Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 analyzed, no information was provided concerning the provision of a certain level of protection to the patient with adapted protective gear such as the casualty bag used by North Atlantic Treaty Organization (NATO; Brussels, Belgium) nations since the Cold War 42 in order to prevent secondary caregivers’ exposure and to mitigate contaminant absorption due to residual contaminants on the patients’ clothes. Decontamination capability information was also lacking. Reference Nozaki, Hurl and Shinozawa37–Reference Yanagisawa, Morita and Nakajima40 These gaps suggest that medical algorithms, protective equipment, and decontamination processes in acute settings in the context of a mass-casualty event due to a chemical attack need to be implemented concurrently.
Most of the events studied occurred years ago (more than 25 years for Japan and seven for Syria). Despite this, and the numerous chemical attacks that took place during the Iran-Iraq war (1983-1988), a lack of publications, applied clinical knowledge, and evidence-based practices still exists when it comes to ensuring in-depth and efficient protection and decontamination for the patient and the clinician. In the literature, studies regarding protection have mostly focused on responders and medical staff PPE. Reference Hick, Hanfling and Burstein43–Reference Ramesh and Kumar45 Very little attention has been paid to patients. Reference Bourassa3,Reference Schulz-Kirchrath9,30,Reference Kaufman46,Reference Bourassa, Bouchard and Lellouche47 To the authors’ knowledge, few studies have investigated the integration of medical devices in PPE Reference Bourassa3,Reference Bourassa12,Reference Bourassa, Bouchard and Lellouche47–Reference Bourassa, Bouchard and Lellouche49 for quicker clinical responses. Reference Bourassa3,Reference Bourassa12 Regardless of the wearer, PPE does not allow for easy access to monitor vital signs or initiate medical interventions such as respiratory and hemodynamic management. It also seems that consideration has yet to be given to populations such as pregnant women, children, and patients with psychiatric, acute, and chronic illnesses. It should be noted that decontaminating a patient is expected to be a complex specialized task best performed by a trained clinician. This can, for example, entail combining decontamination techniques with the safe use of decontaminants and equipment, and most importantly, adjusting patient treatment as required in response to their deteriorating condition or specific injuries (eg, cardiac arrest or open wounds).
Strengths and Limitations
This study’s strength is the exhaustivity of the literature analysis. However, the study also has limitations. Its results may have been subject to a publication bias due to inaccessible classified information that, unbeknownst to the authors, may still exist in Japan or within international organizations such as the World Health Organization (WHO; Geneva, Switzerland), the Organization for the Prohibition of Chemical Weapons (OPCW; The Hague, Netherlands), and the United Nations Office for Disarmament Affairs (UNODA; New York USA). Despite the doubts cast by Rosman, et al regarding the authenticity of the YouTube footage following a chemical attack in Syria, Reference Rosman, Eisenkraft and Milk39 this current study was not able to confirm whether the results were prejudicially biased, which could have induced a selection and an information bias. The studies selected reported data on chemical attacks that occurred more than 10 to 20 years ago. Patient management has evolved, especially with the increased awareness of PPE since the COVID-19 pandemic. Nevertheless, the limited number of studies with a moderate risk of bias as well as the heterogeneity of their methods and results may have hindered the ability of this study to draw any firm conclusion.
Conclusion
This systematic review demonstrates gaps in clinical knowledge and protection and decontamination capabilities concerning the medical extraction of casualties exposed to a chemical attack. Therefore, further research is required to optimize a clinical practice integrating mixed capabilities (protection and decontamination) for the benefit of patients and medical staff.
Conflicts of interest/funding
All authors have completed the Unified Competing Interest form and declare no conflict of interest relevant to this study. Medical Intelligence CBRNE Inc. (also known as MEDINT CBRNE Group) did not provide financial support for the study. Although SB and MD are MEDINT CBRNE Group founders and shareholders, they have no financial interests relevant to the submitted work. MEDINT CBRNE Group is a start-up company that was established in 2017 with support from university entrepreneurship services (Laval and Montreal) and Prince’s Trust Canada. The military expertise that has shaped MEDINT CBRNE Group was developed while serving in the Canadian Armed Forces. The study was partly funded through programs for veterans that assist Canadian Armed Forces members, particularly those released on medical grounds, transition to civilian life (SB). In this instance, funding was dedicated to a doctorate in biomedical and clinical science with a specialty in experimental medicine. Funding sources played no role in the design of the study; the collection, analysis, and interpretation of data; the drafting of the report; or the decision to submit this article for publication. Each MEDINT CBRNE Group member provided their expertise at no cost to the study.
Acknowledgments
The authors are grateful for the support of librarians and technicians from Laval and Montreal Universities (librarians: Frederic Bergeron, Monique Clar; technicians: Marie Bourdeau, Eve Baribeau); Medical Intelligence CBRNE Inc. (François Leger); and for the advice provided by Cochrane Canada Francophone (Marie-Joëlle Cossi). The authors also acknowledge the participation of Mrs. Jessica Thiffault as member of triage team, and Dr. Maude St-Onge, MD for her contribution in the development of the study protocol. The contribution of expertise in CBRNE defense, intelligence, and military medicine made by members of the research team with a military background also constitutes an important value-added of this study. Their service in the Canadian Armed Forces brought priceless value to this work.
Author Contributions
Study Concept and Design: Stephane Bourassa (SB), Emmanuelle Paquette-Raynard (EPR), Jacinthe Leclerc (JL), Philippe Jouvet (PJ).
Data Acquisition: Emmanuelle Paquette-Raynard, Stephane Bourassa, Daniel Noebert, Pelumi Samuel Akinola, Jason Marseilles, Jessica Thiffault, Marc Dauphin, Philippe Jouvet.
Systematic Review Validation: Emmanuelle Paquette-Raynard, Stephane Bourassa, Daniel Noebert, Samuel Akinola, Jason Marseilles, Marc Dauphin, Philippe Jouvet.
Data Analysis and Interpretation: Stephane Bourassa, Emmanuelle Paquette-Raynard, Jacinthe Leclerc, Philippe Jouvet.
First Draft of the Manuscript: Stephane Bourassa.
Critical Revision of the Manuscript for Important Intellectual Content: Emmanuelle Paquette-Raynard, Daniel Noebert, Marc Dauphin, Pelumi Samuel Akinola, Jason Marseilles, Philippe Jouvet, Jacinthe Leclerc.
Study Supervision: Jacinthe Leclerc, Philippe Jouvet.
Accountability: Philippe Jouvet (PJ), Jacinthe Leclerc (JL), and Stephane Bourassa (SB) take responsibility for the content of the manuscript, including the data and analysis. The lead author (JL) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, and that no important aspects of the study have been omitted.
Supplementary Materials
To view supplementary material for this article, please visit https://doi.org/10.1017/S1049023X22000401







